Lactose intolerance
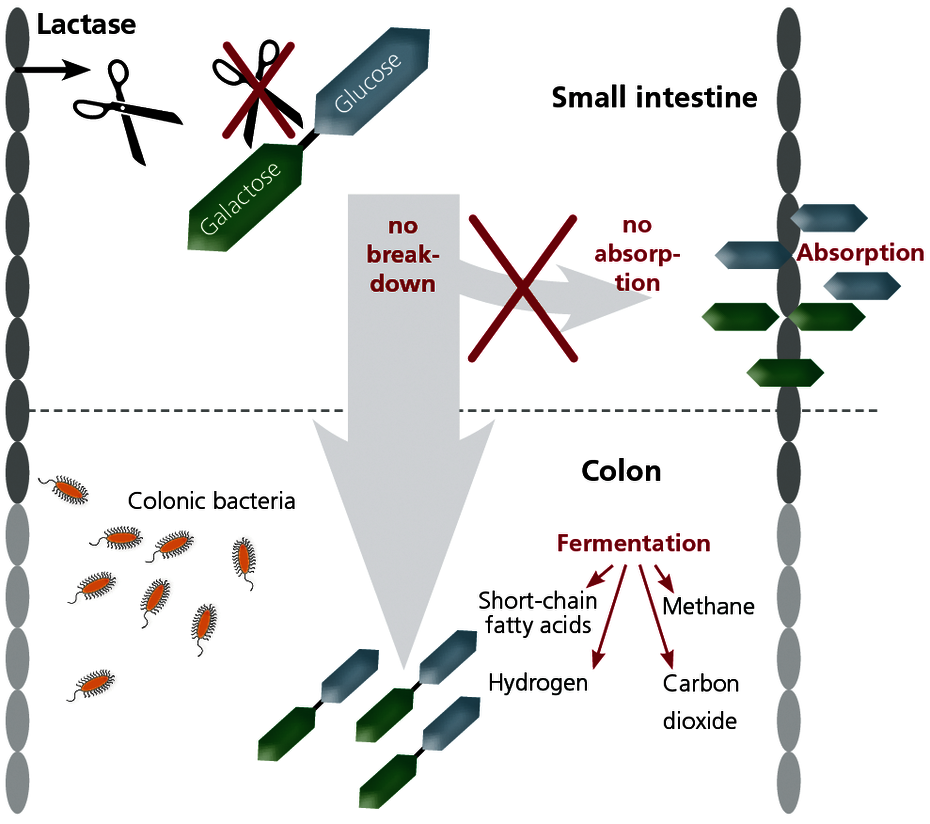
Lactose (milk sugar) is a disaccharide contained in many foods, which is normally broken down into its components galactose and glucose in the small intestine by the enzyme lactase. Only these monosaccharide molecules can be absorbed through the wall of the small intestine.
If the enzyme lactase is deficient, lactose cannot be broken down and therefore cannot be absorbed. The lactose then ends up in the colon undigested, where it is fermented by the intestinal bacteria that are normally there. Lactose intolerance is therefore due to an enzyme deficiency and cannot be confused with a milk allergy, where the immune system reacts to components in milk.
What causes the symptoms in lactose-intolerant patients?
The undigested lactose molecules result in bacterial fermentation products in the colon such as carbon dioxide (CO2), short-chain fatty acids, hydrogen, and methane. The lactose molecules are also active osmotically. The gases resulting from these processes and the osmotically-induced influx of water in the colon cause the following symptoms:
- Bloating
- Flatulence
- Meteorism (drum-like distention of the abdomen)
- Cramping stomach pain
- Diarrhoea
The reduced absorption of glucose in the small intestine can also temporarily induce symptoms of fatigue.
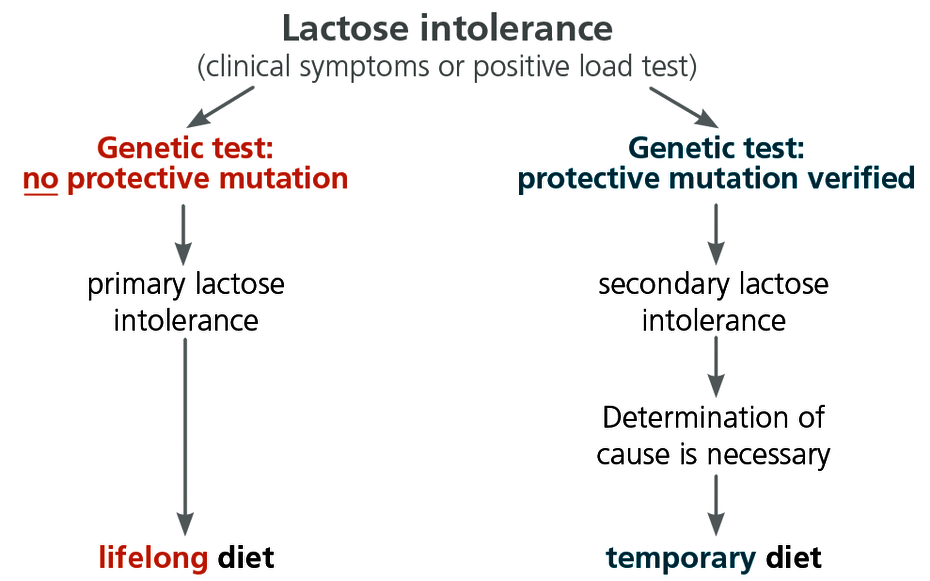
With lactose intolerance, the genetic primary form and a form that is acquired secondarily must always be differentiated, as this is critical for the treatment.
Primary (adult) lactose intolerance is the most common enzyme deficiency worldwide
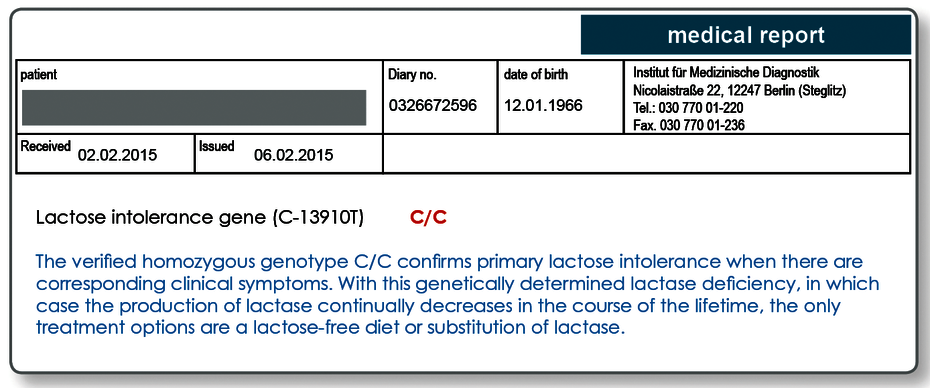
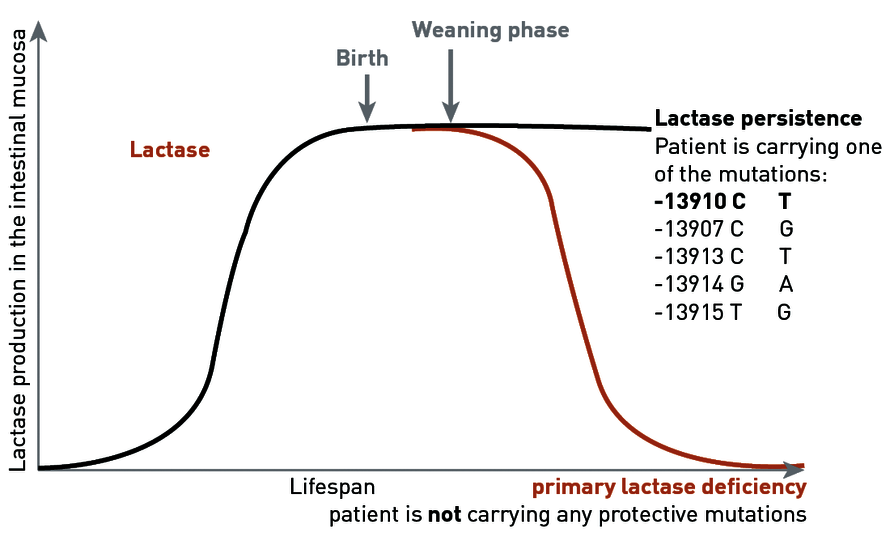
As with all mammals, the production of lactase in humans decreases after weaning through withdrawal of the mother's milk. In populations that carry out intensive dairy farming, protective mutations developed approximately 7,500 years ago, ensuring lifelong lactase persistence for the mutation carrier. In Europe, as an adaptation to the lifelong consumption of lactose, a cytosine (C → decrease in lactase production) at the position -13910 in the lactase gene in the DNA has been exchanged for a thymidine (T → lactase persistence).
Around 75% of the German population have this gene variant, which is why verification of differentiation of primary and secondary lactose intolerance has become established in Germany.
For patients that do not carry this protective mutation, lactase production continues to decrease in the course of life. It is therefore assumed that 25% of the population develop lactose intolerance during their lives.
Higher sensitivity thanks to new analytical methods
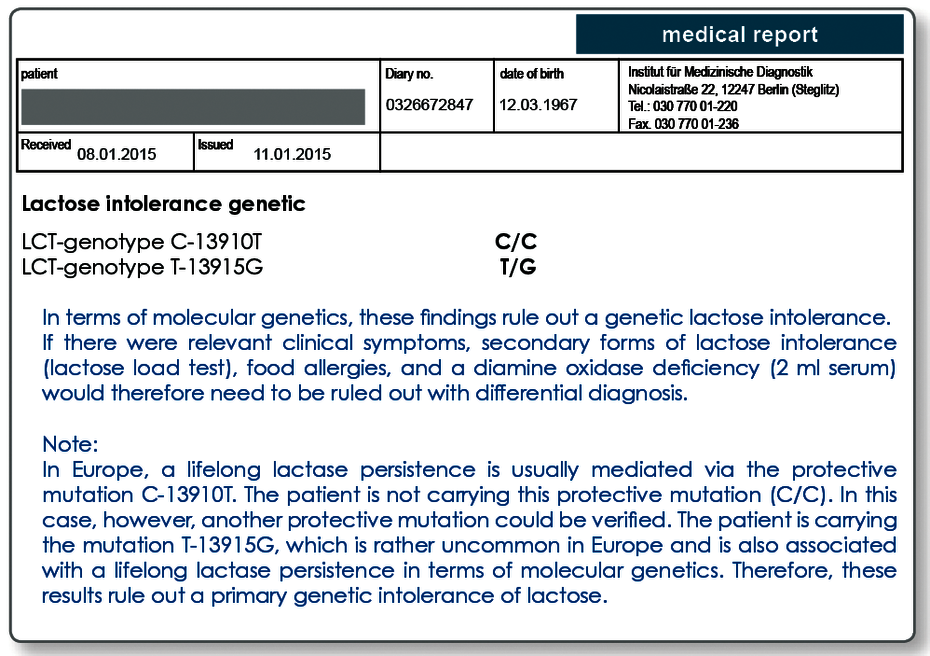
Today, we know that the figure of 25% was overestimated, as the mutation -13910 C → T that is usually investigated is not solely responsible for the persistence of lactase production. In parallel with the development in Europe, an adaptation to the lifelong consumption of milk sugar has taken place in other regions also. Other genetic variants in the lactase gene that are associated with lactase persistence are the polymorphisms -13907 C → G, -13913 C → T, -13914 G → A, and -13915 T → G.
These genetic variants appear after all in approximately 10% of cases that are examined in our lab. These additional mutations are therefore also present in the German population. This fact has resulted in the analytical technology being changed over to the cutting-edge DNA sequencing, and therefore all responsible mutations are detected unequivocally. As a consequence, a (further) protective mutation can be determined for an additional 10% of the patients.
This means that around 15% of the population in Germany suffer from a primary (genetically-determined) lactose intolerance. Genetic testing today allows the genetic disposition to be reliably attributed to a primary (genetic) lactose intolerance.
With the secondary form of lactose intolerance, the production of lactase is not reduced because of genetics but as the result of another underlying disease.
A secondary lactase deficiency can manifest itself in damage to the epithelium of the small intestine (the place where lactase is synthesised) e.g. when cytostatic or antibiotic treatment is given, or with patients who have coeliac disease or Crohn's disease.
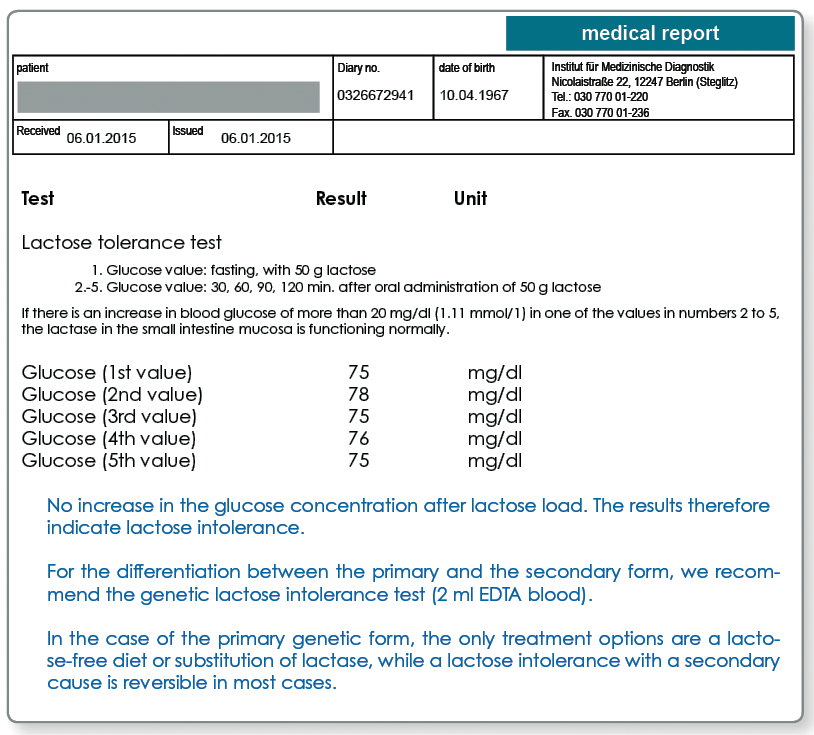
A lactase deficiency with a secondary cause is only temporary and is reversible after the intestinal epithelium is regenerated. The lactose load test is used diagnostically to verify a current lactose intolerance.
Only the genetic test can differentiate between primary and secondary lactose intolerance
The genetic testing can be used to differentiate between primary and secondary lactose intolerance. The lactose intolerance genetic test is therefore recommended as follow-up testing in the case of all positive results in the lactose load test.
This is of great therapeutic importance because, for patients with the primary form of lactose intolerance, the only possible treatment options are a lactose-free diet or a diet low in lactose, or else to take lactase preparations for life. For patients with a secondary form of lactose intolerance, however, this treatment is only necessary until the intestinal epithelium has regenerated, following clarification of the cause and treatment of the underlying disease. A lifelong lactose-free diet is not necessary in the case of secondary lactose intolerance.
If there are significant clinical symptoms, the lactose-intolerance genetic test can also be used in differential diagnosis of lactose intolerance, as an alternative to the load test that is non-stressful to the patient.
Chronic inflammatory bowel diseases are often the cause of a secondary lactase deficiency
As potential causes of a secondary lactase deficiency, first and foremost, apart from allergies to milk products and relevant dysbioses, chronic inflammatory bowel diseases should be ruled out. If Crohn's disease or ulcerative colitis is suspected, determination of the genetic markers NOD2 and ATG16L1 can be helpful. The verifiable ASCA that accumulate with Crohn's disease and pancreatic acinar cell autoantibodies or the goblet cell autoantibodies and pANCA associated with ulcerative colitis can be determined with laboratory tests.
Detailed information on the diagnosis of coeliac disease
Sample materials required
Genetic lactose intolerance test: 2 ml EDTA blood.
Transport of the blood sample to the laboratory is not time critical and can be done by mail. For genetic testing, we require the signed consent of the patient.For further questions please contact us by phone at 030 77001 220.
Lactose load test:
- Fasting blood collection to determine the blood glucose (citrate/sodium fluoride tube)
- Patient drinks 50g lactose in 400 ml water, or 2g lactose/kg body weight
- Further collections of blood after 30, 60, 90, and 120 min to determine the blood glucose (1 citrate/sodium fluoride tube each)
Literature
- Imtiaz et al. (2007). The T/G 13915 variant upstream of the lactase gene (LCT) is the founder allele of lactase persistence in an urban Saudi population. J Med Genet. 44(10):89.
- Ledochowski et al. (2003). Laktoseintoleranz. J Ernährungsmed. 5(1):7-1.
- Mattar et al. (2012). Lactose intolerance: diagnosis, genetic, and clinical factors. Clin Exp Gastroenterol. 5:113–121.
- Nilsson &Olsson (2008). Simultaneous genotyping of the three lactose tolerance-linked polymorphisms LCT -13907C>G, LCT -13910C>T and LCT -13915T>G with Pyrosequencing technology. Clin Chem Lab Med. 46(1):80-4.
- Tishkoff et al. (2007). Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet. 39(1):31-40.