Toxicological and allergological aspects of sensitivities against endoprostheses
Medical progress has made treating damaged joints with endoprosthetic joint replacements possible. In Germany, approximately 400 000 treatments of this kind are performed each year. Of major importance are replacements of hip and knee joints, while either chromium-cobalt-molybdenum alloys, high-quality steel or titanium is used. For sliding surfaces, grating surfaces, and tribological pairings, plastic compounds are used as well. However, pure metal-on-metal pairings are also common. Information regarding endoprostheses’ revision rate is, until today, only partly available, since there is no reliable and systematic analysis of quality and results at hand. Assumed figures for hip endoprostheses are at around 5-7 % and for knee endoprostheses revision rates are at around 1.3-3.4 %. Since the end of October 2011, the German Society of Orthopaedics and Orthopaedic Surgery (Deutsche Gesellschaft für Orthopädie und Orthopädische Chirurgie, DGOOC) has been administering a registry on German endoprostheses (EPRD) on a trial basis.
There are many reasons, why revisions become necessary. Next to the consumption of slide-promoting plastics and infections, the loosening of prosthesis compartments plays a key role as a possible cause for inflammatory processes in the tissue surrounding implants. Recently, systemic “sideeffects”, such as local or disseminated eczema, disorders of wound healing, impacts on systemic inflammatory processes, or potentially toxic effects on the organism due to released metal ions, have been discussed increasingly.
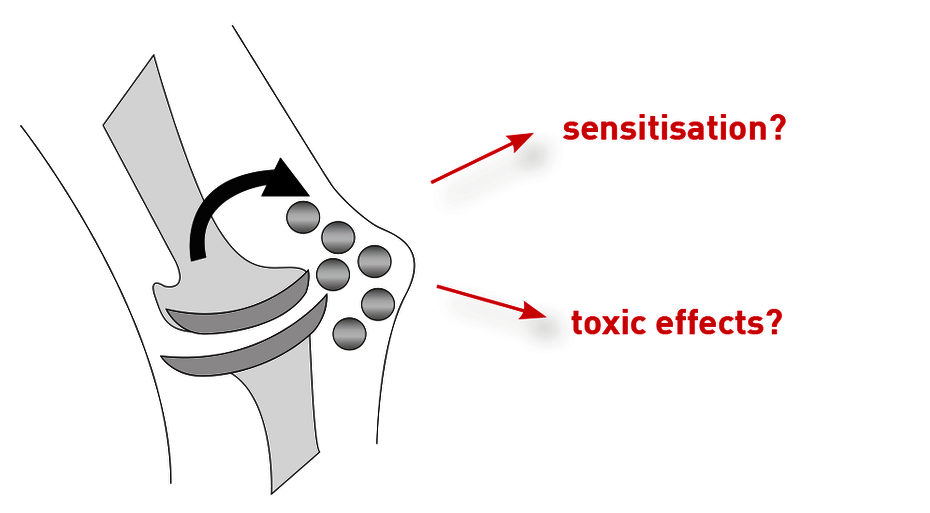
Allergy-induced sensitivity against prostheses
A type 4 sensitisation (delayed-type allergy) against metallic ingredients of implants and constituents of bone cements may cause “sensitivity against endoprostheses”. Painful synovia inflammations, osteolyses, and the endoprosthesis’ aseptic loosening are possible local symptoms of an allergy-induced sensitivity against implants. Due to the systemic character of the cellular immune response, possible other manifestations of an allergy against incorporated materials are skin reactions, such as local or disseminated eczema, but also disorders of wound healing, and the inducing of other systemic inflammatory phenomena. Available data is inconclusive. Various studies show that despite an existing sensitisation against metals, some metal implants are well tolerated. However, the fact that, especially if an allergy against mentioned materials is present prior to the implantation, inflammations may occur after lymphocytes had contact with the allergen in peri-implant tissue, is uncontested. It is worth noting that the Australian implant registry mentions metal allergies as the reason for revisions of metal-metal prostheses in 5.7 % of cases.
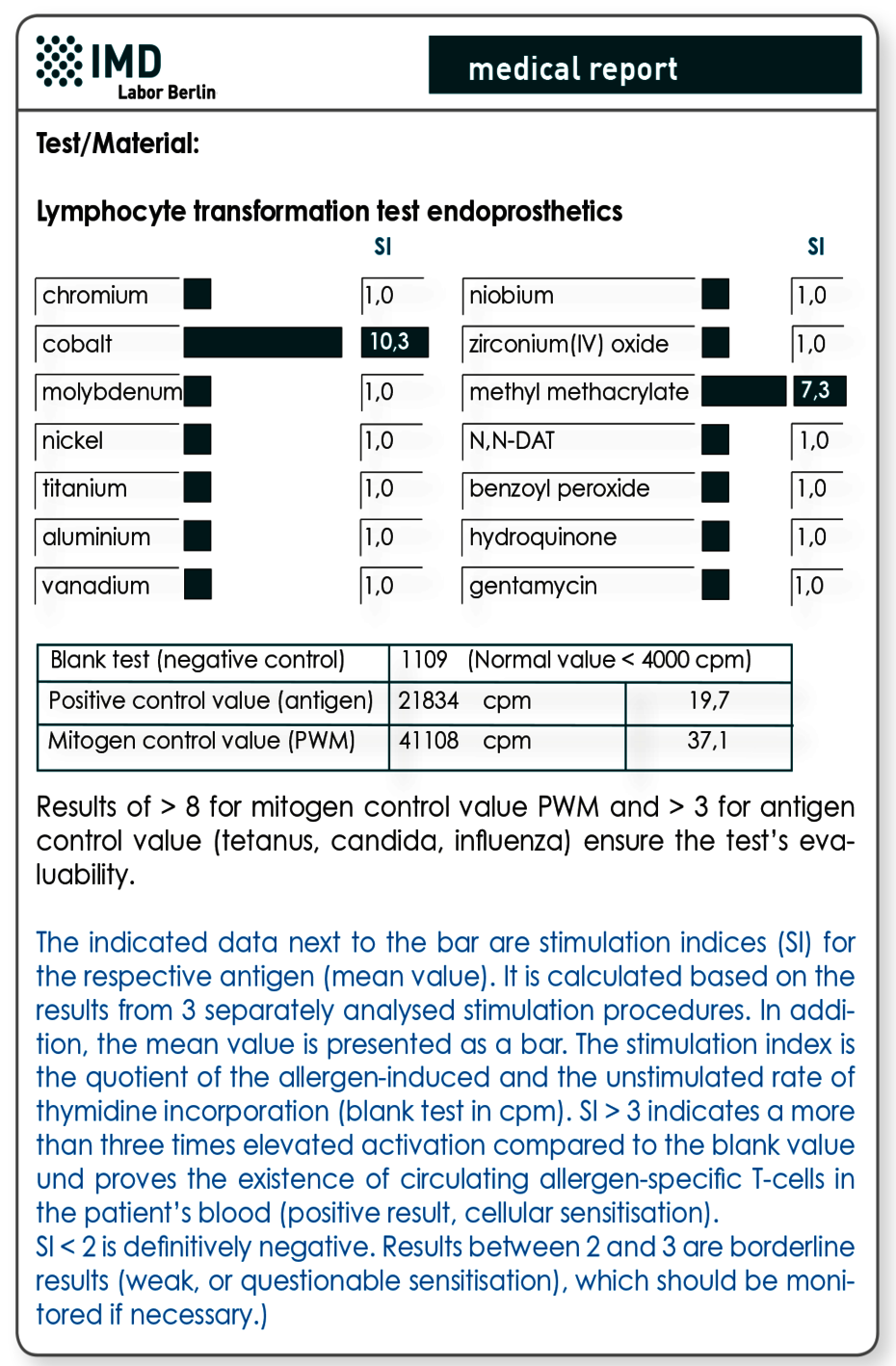
Exclusion of an existing sensitivity is achieved using the lymphocyte transformation test (LTT)
A skin test (epicutaneous test) should not be used for prophylactic testing, since here an iatrogenic sensitisation due to the test itself is possible. The latter might have disastrous consequences, because a sensitisation would not be visible (yet) in the test results. The allergic reaction would then unfold with the material’s implantation. Furthermore, the lymphocyte transformation test has proven to be more sensitive for systemic sensitisations (contact with metal not via skin), since the systemic, not the local, sensitisation is analysed. In particular for prophylactic testing, a combination profile has been developed, which, next to metals potentially occurring in implant, also includes ingredients of cements. Those cover acrylates, the polymerisation initiators benzoyl peroxide and hydroquinone, as well as gentamycin, which is often included in cements.
Material (LTT)
2x10 ml heparin blood + 5 ml serum
Costs (LTT)
Please obtain the costs for the analysis from the pdf-document.
Toxicological sensitivity due to release of metal ions from the implant
Endoprostheses may release significant amounts of metal ions and particles due to corrosion and/or abrasion. Studies show that significant metal release correlates with a high probability for a revision. However, chronically increased metal loads can not only cause sensitivities against the implant itself, but may furthermore have a toxicological impact on the entire organism. A case of a patient has been published only recently, where a chromium and cobalt burden stemming from a hip implant lead to a severe retinopathy. Additionally, the commonly used cobalt is a known mutagen. Possibly toxic effects of the released metals differ from individual to individual and occur independently from sensitisations.
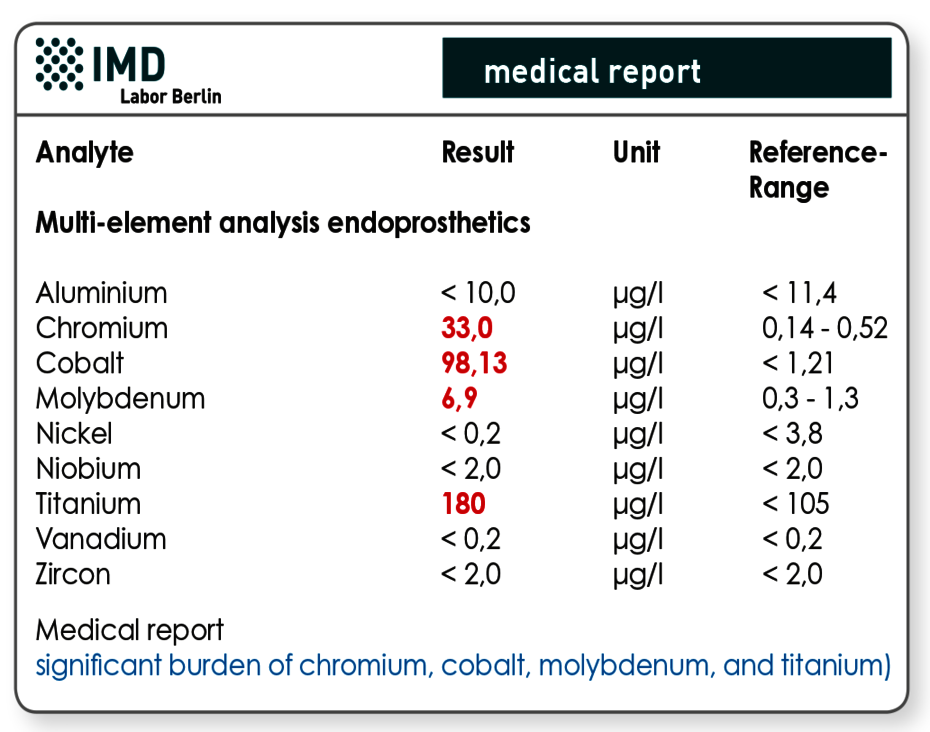
Multi-element analysis is used to determine the metallic burden
Metal concentrations in EDTA whole blood are especially conclusive regarding possible toxic effects and for the detection of changes over time. Just like the LTT profile, the profile (MEA endoprosthetics) entails all relevant metals that are used in endoprostheses.
Material (Multi-element analysis)
2 ml EDTA blood
Costs (Multi-element analysis)
Please obtain the costs for the analysis from the pdf-document.
Literature
- Mitteilung der Kommission „Methoden und Qualitätssicherung in der Umweltmedizin des Robert-Koch-Institut“, „Qualitätssicherung beim Lymphozytentransformationstest“ – Addendum zum LTT-Papier, Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz 2008 ; 51:1070–1076
- Apel et al., Cobalt-chromium toxic retinopathy case study. Documenta Ophthalmologica 2013;126: 69-78
- Basko-Plluska JL et al. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011; 22:65-79.
- Frigerio E. Metal sensitivity in patients with orthopaedic implants:a prospective study. Contact Dermatitis. 2011; 64:273-9
- Hallab J et al. Lymphocyte responses in patients with total hip arthroplasty Journal of Orthopaedic Research 23 (2005) 384–391
- Meftah et al., Early corrosion-related failure of the rejuvenate modular total hip replacement. J Bone Joint Surg Am. 2014; 96: 481
