Titanium Sensitivity
Titanium sensitivity is not an allergy
If compared to other metals, titanium possesses a relatively low allergenic potency. This is due to the fact that because of their high tendency to oxidise, titanium ions originating from implants are oxidised immediately after release. Contrary to metal ions, oxidised titanium particles are incapable of becoming allergens by modifying proteins, which means they do not have any haptic effect.
Titanium sensitivity is the consequence of an increased propensity to inflammation
Mucositis and peri-implantitis are complications occurring in a sub-group of patients. The most common cause of individual hypersensitivity to titanium is an exaggerated pro-inflammatory reactivity of tissue macrophages. These “cleanup-cells” phagocytose titanium oxide particles, which are released in the area surrounding implants (particular debris). Macrophages reacting with the release of pro-inflammatory cytokines, in particular with TNF-α and Interleukin-1, to contact with titanium oxide particles, is a physiological effect. However, the extent of the immune response is highly individual. The intensity of the cytokines’ release depends on existing genetic variants (polymorphisms) of the involved pro-inflammatory (IL-1 und TNF-α) and anti-inflammatory (IL-1- receptor antagonist IL- 1RN) mediators.
In contrast to all other metals, titanium-specific lymphocytes are not involved, which explains negative LTT and epicutaneous test results.
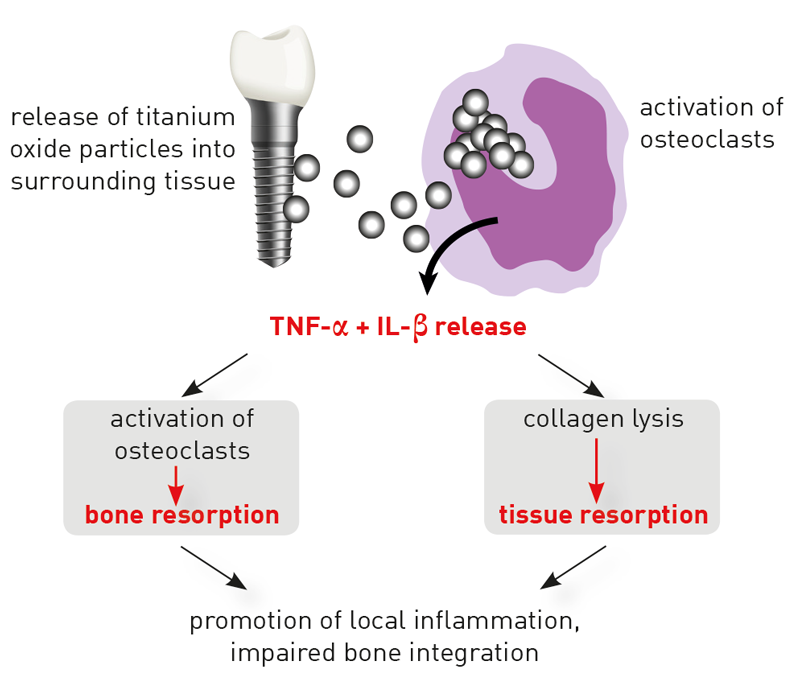
The titanium stimulation test detects cytokine responses after contact with titanium oxide
The titanium stimulation test has been designed and validated to answer this exact question1 . The whole blood stimulation test examines whether a patient‘s monocytes/macrophages react with an excessive inflammatory response to the exposition with titanium particles. A reaction of this kind is indicated by an increased release of TNF-α and/ or IL-1β. In patients with positive test results, delayed or impaired healing of titanium dental implants is to be explained by the fact that macrophages in the area surrounding the implant show hyperactive responses when facing released titanium particles. Thereby, they induce primarily local, and potentially also systemic inflammations.
With an increased genetic degree of inflammation, the risk for a titanium-associated inflammatory process/loss of implant rises accordingly
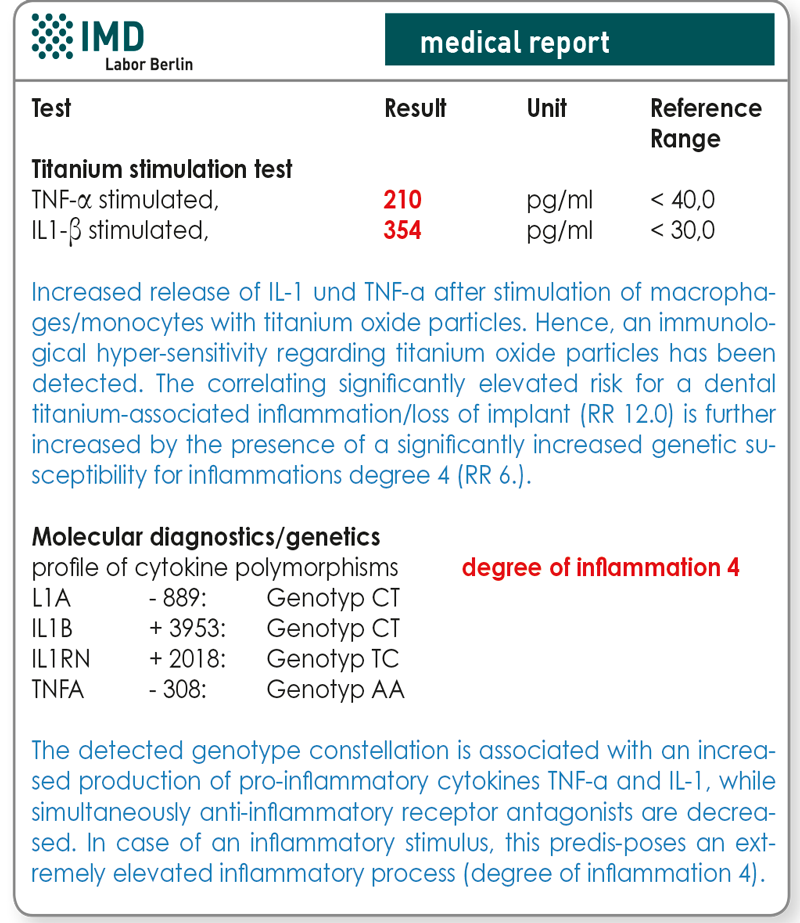
A variety of studies has proven the interrelation between peri-implantitis, or respectively loss of implant, and functionally relevant polymorphisms in the genes of cytokines IL-1, IL-1RN and TNF-α2,3,4. Reported gene polymorphisms of TNF-α, IL-1 and IL1-RN can be detected using laboratory tests. This molecular genetic procedure has the advantage that current inflammatory processes or immunosuppressive therapies do not affect it. Using genetic tests allows for the allocation of a certain degree of inflammation to the detected combination of alleles. Patients with degree 3-4 are considered high responders and are thus risk patients for titanium-associated inflammatory processes/loss of implants (tab. 1). These polymorphisms are clinically relevant since patients possessing high responder polymorphisms have an increased susceptibility for the development of periprosthetic bone loss5,6.
The degree of inflammation and positive results from titanium stimulation tests are significant and independent additive risk factors
In a study initiated by the German Society for Environmental Dentistry (Deutsche Gesellschaft für Umwelt-Zahnmedizin, DEGUZ), the prognostic value of both analyses has been confirmed7 . If compared to a control group (68 patients having implants for more than 10 years with unproblematic healing), patients with loss of implant and without exposure to stress during healing (n=14) and patients with loss of implant after stress (n=29) had a significantly higher titanium oxide-induced in vitro release of TNF-α and IL-1β (p<0.0001). Positive results of titanium stimulation tests constitute a risk factor for titanium-associated inflammations/loss of implant, which is elevated twelvefold and independent of age, gender and smoking habits. Besides that, the number of risk polymorphisms and the resulting genetic degree of inflammation influence the probability of a loss of implant significantly (p=0.046). With an increasing degree of inflammation, the risk of a titanium-associated inflammation/loss of implant increases as well (tab. 1).
What does a positive result of a titanium stimulation test or an elevated degree of inflammation imply?
These tests executed prior to implantations, since indicative results in one of both tests signal a predisposition for titanium-associated inflammatory processes. Positive results should not be interpreted as an allergy, which would require the allergen’s general avoidance. Therefore, positive titanium stimulation test results or high responder gene constellations as such do not constitute an absolute contra-indication for a titanium implant. However, alternatives (such as ceramic implants) should be carefully considered and prophylactic measures intensified accordingly (intensified prophylaxis, no immediate implant placement, sanitation of the infectious source, smoking cessation, anti-inflammatory measures). Until four weeks after the implantation, any type of therapies stimulating the immune system should be omitted. Where applicable, anti-inflammatory measures at the time of implantation can be beneficial.
Material
Titanium stimulation test: 10 ml heparin blood
Sample receipt within 24 hrs has to be ensured. Within the Berlin city area, we offer a courier service (+49 (0)30 7701- 250). For collections beyond Berlin, please contact our complimentary courier service (+49 (0)30 77001-450).
Genetic susceptibility for inflammations: 2 ml EDTA blood or smear-test sample of oral mucosa
Transport to the laboratory is not time-sensitive and can be sent by mail.
Costs
Please obtain the costs for the analysis from the pdf-document.
Should prior to an implantation or in case of suspected titanium sensitivity an LTT be administered additionally?
Type 4 sensitisations regarding titanium are extremely rare, which is due to titanium’s high tendency to oxidise, mentioned above. Hence, in the given context, the LTT is clearly less important than the other two tests. Regarding type 4 sensitisations, contaminating metals are likely to be more relevant. Some titanium implants contain traces of nickel, vanadium, or aluminium. Therefore, a screening profile complementary to the LTT has been developed, which allows for the additional testing for these three metals alongside with titanium.
Material and costs for the LTT
20 ml heparin blood and 5 ml whole blood. Sample receipt within 24 hrs has to be ensured.
Profile LTT titanium alloy contains: Titanium, nickel, vanadium, aluminium
Please obtain the costs for the analysis from the pdf-document.
Would you like to see a presentation on the matter?
In our video archive, you can find a free presentation on this topic. Access is free und possible without prior registration.
Literature
- Dörner T, von Baehr V et al. Implant-related inflammatory arthritis. Nature Clin Pract Rheumatol. 2006;2:53).
- Jansson, H et al. (2005): Clinical consequences of IL-1 genotype on early implant failures in patients under periodontal maintenance. Clin Implant Dent Relat Res 7(1): 51
- Laine, M. et al. (2006):IL1RN gene polymorphism is associated with peri-implantitis. Clin Oral Implants Res 17(4): 380
- Montes, C.C. et al. (2009): Analysis of the association of IL1B (C+3954T) and IL1RN (intron 2) polymorphisms with dental implant loss in a Brazilian population. Clin Oral Implants Res 20(2):208
- Feloutzis, A. et al (2003): IL-1 gene polymorphism and smoking as risk factors for periimplant bone loss in a well-main tained population. Clin Oral Implants Res 14:10
- Gruica, B. et al. (2004): Impact of IL-1 genotyp and smoking status on the prognosis if osseointegrated implants. Clin Oral Implants Res 15(4): 393
- Jacobi-Gresser et al. (2013): Genetic and immunological markers predict titanium implant failure: a retrospective study. Int J Oral Maxillofac Surg. 42 (4) : 537
