Lymphocyte transformation test (LTT)
Can dental restoration materials cause allergies?
Metals and acrylates (plastics) as well as many other components present in root canal filling materials or cements may be potential allergens.
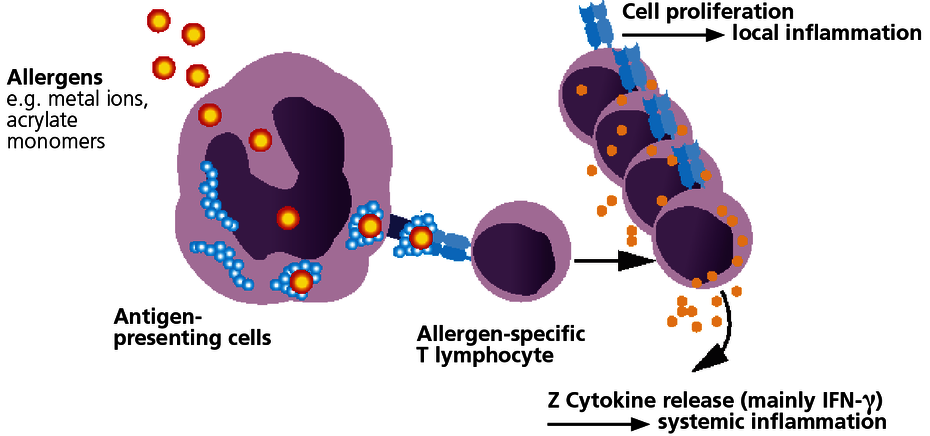
With few exceptions, sensitisation to dental materials is based on type IV sensitisations (late-type allergies). With this type of allergy, specific T lymphocytes form which identify as foreign the allergen or the body’s own protein that has been modified by the allergen (hapten effect). In sensitised patients, the immune system responds to contact with the relevant allergen with immune activation. This can be expressed as local symptoms but may also cause or amplify systemic inflammatory reactions.
Type IV sensitisation to an allergen then exists once the patient has developed allergen-specific T lymphocytes. This is verified with the LTT.
When does intolerance to dental materials caused by an allergy have to be considered?
Local signs include stomatitis, lichen ruber planus, gingivitis or periodontitis. They are not, however, obligatory because the oral mucous membranes are immunologically less reactive. Because of the special immunological characteristics of the oral mucosa, local symptoms such as a burning tongue or jaw pain and toothache are therefore not associated with a morphological correlate.
Because immune reactions have a systemic character as a matter of course, with type IV allergies many generalised symptoms may be present or be increased. These non-specific inflammatory symptoms include exhaustion, sleep disorders, depressed mood, muscle pain, arthralgia (fibromyalgia), paraesthesia, headaches, migraines or even neuralgia.
It is also known that prolonged exposure to metal ions (including mercury, gold, nickel) can trigger autoimmunity in sensitised patients.
The LTT can detect allergen-specific T lymphocytes.
The LTT is a laboratory method to detect a specific cellular sensitisation. The test is based on the principle of allergen-specific induction of cell division in lymphocytes after contact with their ‘matching’ allergen. A positive reaction in the LTT indicates the presence of allergen-specific lymphocytes (memory cells) in the patient’s blood.
There are two questions that can be answered with the LTT:
- Is it necessary to replace the existing dental restoration material?
Using the LTT, a correlation to the material can be verified or excluded if symptoms develop after the dental restoration material is applied (curative clinical question). - What materials can be used or should not be used?
Using the LTT, existing sensitisations to any of the components of a new dental restoration material can be excluded prior to its use (preventive clinical question).
Which materials can be tested for using the LTT?
In principle, almost all dental restoration materials can be tested in the LTT provided a cytotoxic effect or non-specific activating factors can be excluded in the laboratory.
For frequently occurring clinical questions, the following profiles have been developed in which the known individual sensitising allergens undergo standardised testing.
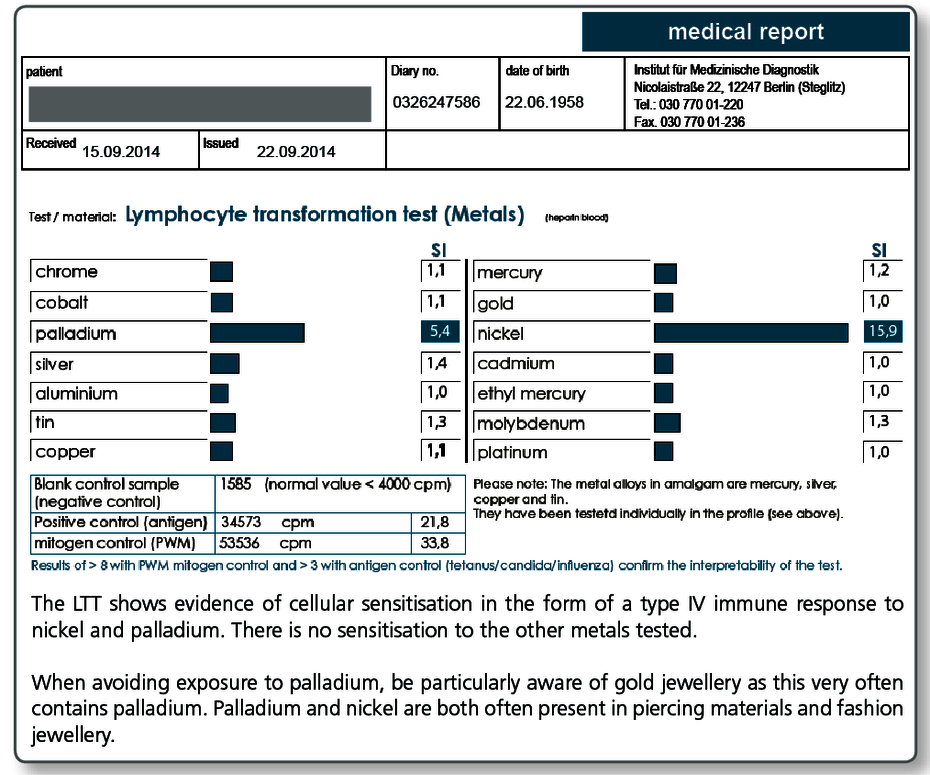
| LTT Metals | Gold, nickel, palladium, chromium, cobalt, molybdenum, aluminium, platinum, cadmium, mercury, copper, silver, tin, ethylmercury |
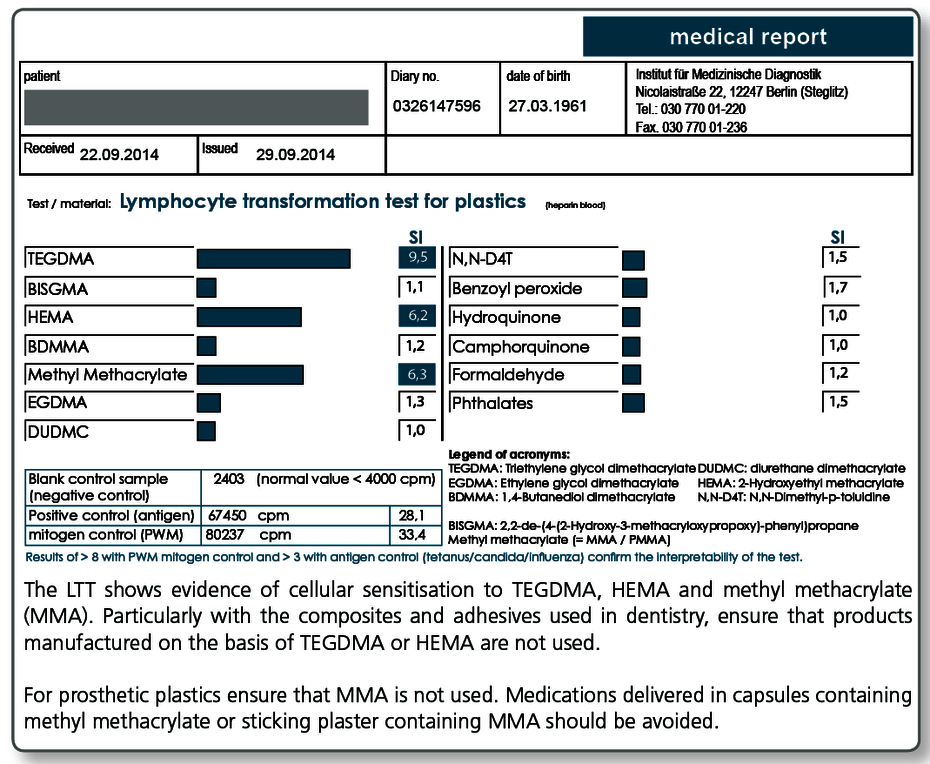
| LTT Plastics | Methyl methacrylate (MMA/PMMA), TEGDMA, BISGMA, BISDMA, HEMA, diurethane dimethacrylate, ethylene glycol dimethacrylate, butanediol-1-4-methacrylate, N,N-dimethyl-4-toluidine, benzoyl peroxide, hydroquinone, camphorquinone, phthalates, formaldehyde |
| LTT Combination Profile (Dentalcheck) | Gold, nickel, palladium, chromium, cobalt, platinum, mercury, copper, silver, tin, methyl methacrylate (MMA/PMMA), HEMA, TEGDMA, BISGMA |
| LTT Gold Alloys | Gold, silver, platinum, copper, palladium, tin, gallium, indium, iridium, rhodium, tantalum, ruthenium |
| LTT Amalgam | Amalgam components and organic mercury compounds: mercury, copper, silver, tin (amalgam), ethylmercury, phenylmercury, methylmercury |
| LTT Root Canal Filling Materials | Raw gutta-percha, balsam of Peru, eugenol, polydimethylsiloxane, silicone oil, bismuth oxide, silver, turpentine oil, colophonium, triethanolamine, peanut oil, paraformaldehyde, bisphenol A, epichlorhydrin |
| LTT Ceramic + Cements | Vanadium, aluminium, titanium, cobalt, chromium, barium, silicon, cerium, boron, manganese, antimony, phosphate cement (Harvard), glass ionomer cement (Ketac-Bond) |
| LTT Native Materials | Testing of a range of selected materials that can also be sent to us in the laboratory. |
Please refer to the detailed description regarding the diagnosis of titanium intolerance.
Are there cost-effective combination profiles?
A combination profile (Dentalcheck) was developed specifically for preventive testing which includes the most important metals and acrylates and covers the following problematic areas:
- Gold alloys (gold, silver, palladium, platinum, copper and tin)
- NPM alloys (chromium, cobalt, nickel)
- Amalgam (mercury, silver, copper, tin)
- Prosthetic plastics (MMA/PMMA)
- Composites and cements containing acrylates (HEMA, TEGDMA, BISGMA)
Can native and custom materials also be tested?
Yes, if the materials are sent to the laboratory along with the blood sample. This procedure has proven itself particularly useful for complex materials such as cements, composites, prosthesis materials, bone graft substitute materials, root canal filling materials (also points) as well as intraoral harvested metal and plastic chips and shavings.
Negative test results for the materials exclude sensitisation to any of the components (declared or not declared). Materials that test positive in the LTT must not be used under any circumstances because there is a type IV sensitisation to at least one of the allergens present.
Note: Native materials frequently used in dentistry are kept in stock in the laboratory. Please call us under +49 30 77001-220 and we will send you this list per fax.
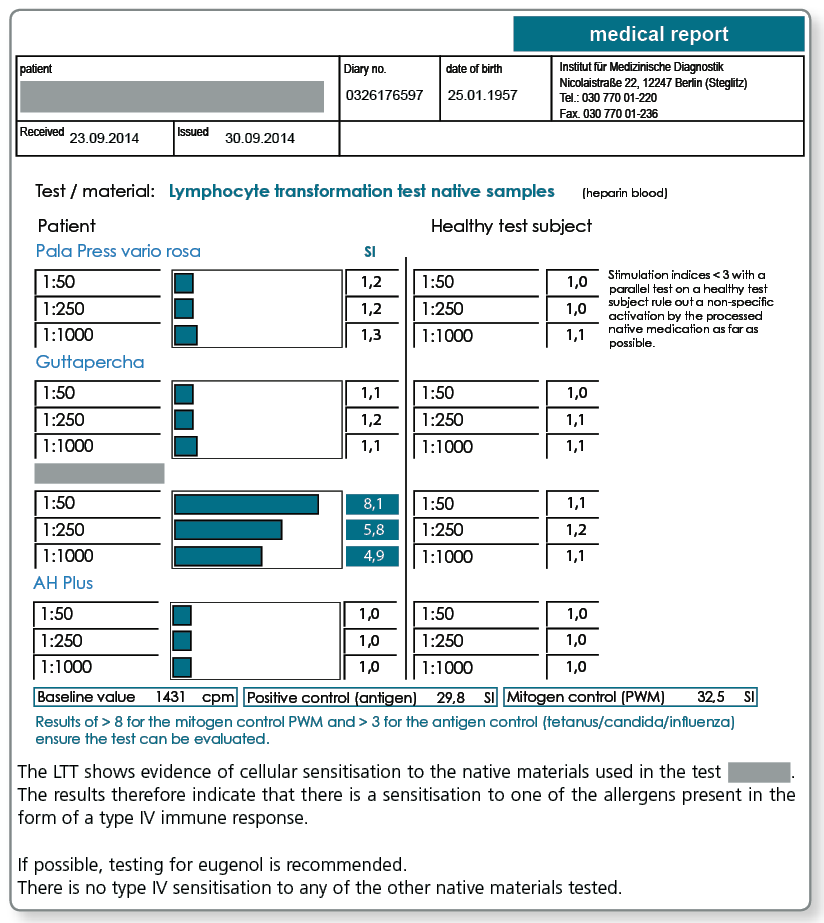
LTT Root Canal Filling Material
There may be allergic sensitisations to gutta-percha but also to other components present in sealers. The known allergens are included in the LTT Root Canal Filling Material profile.
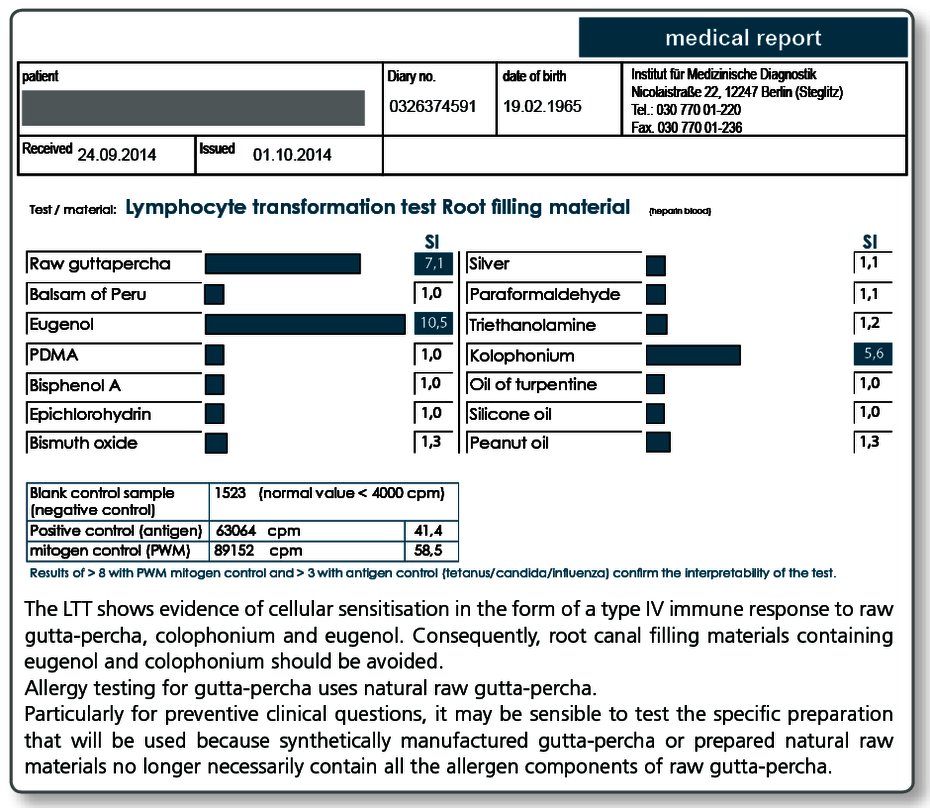
Allergy testing for gutta-percha uses natural raw gutta-percha. Particularly with preventive queries it can be useful to test the specific gutta-percha preparation (points as well) to be used as native material in the LTT, because, for example, synthetically produced gutta-percha or processed natural raw materials no longer necessarily contain all the allergen components present in raw gutta-percha.
To illustrate the importance of the components included in the LTT profile, the (known) important components of commonly used temporary or permanent root canal filling materials are listed below.
The ingredients were taken from the material safety data sheets or the product information. We do not assume any liability for the correctness and completeness of this list.
| N2 Endodontic Cement® | ingredients include paraformaldehyde, titanium oxide, zirconium oxide (powder) and eugenol, rose oil, lavender oil, peanut oil (liquid) |
| Endomethasone® | ingredients include thymol iodide, zinc oxide, hydrocortisone acetate (powder) and eugenol (liquid) |
| AH Plus® | bisphenol A diglycidyl ether, bis [4-(2,3-epoxypropoxy)phenyl]-methane, PDMS |
| AH26® | ingredients include bismuth oxide, methenamine, titanium oxide, silver |
| Aptal-Harz Wurzelfüllung® | ingredients include zinc oxide, colophonium (powder), eugenol, balsam of Peru, turpentine oil (liquid) |
| Rocanal Permanent Vital R2® | ingredients include aniseed oil, phenylphenol (powder), eugenol, colophonium, castor oil (liquid) |
| Hermetic® | ingredients include zinc oxide, zinc stearate, zirconium(IV) oxide (powder), eugenol, balsam of Peru (liquid) |
| Super EBA® | ingredients include eugenol (liquid), zinc oxide, aluminium oxide (powder) |
| Apexit Plus® | ingredients include calcium hydroxide, calcium oxide, colophonium |
| Gutta-percha points | depending on the brand, contain zinc oxide, purified gutta-percha, colophonium (1–4.1%), heavy metals 1.5–30%, possibly pigments, food dyes |
What are the consequences of a positive LTT result?
A positive result in the LTT indicates that there is a type IV sensitisation to the allergen concerned. Materials that contain this allergen should not be used in future (preventive statement). For curative issues, ensure that neither an allergy test (nor the epicutaneous test) is used to verify the direct causal relationship with the existing symptoms.
With a verified sensitisation, it must be carefully weighed up whether the particular problem material should be removed and replaced. What is critical here is the clinical symptoms rather than just a positive test result. Other sources of exposure must be primarily or simultaneously eliminated (detailed information about other sources of exposure can be found in the LTT results).
In an individual case with verified sensitisation and unclear clinical significance, the causal relationship between the sensitisation and the clinical manifestation can be examined in more detail by subsequently carrying out effector cell typing.
What do you need for the LTT?
For each examination profile, 20 mL heparin and 5 mL whole blood are required for serum extraction.
The collection and shipping material is provided by the laboratory free of charge. The blood collection sets are available from all the usual manufacturers.
Please contact our staff in the regional practice support team or our service personnel in the laboratory and they will happily answer all your questions about organising the LTT.
How is the blood collected?
Normally dentists send their patients to their general practitioner with the blood collection set and the completed request form, and he or she then collects the blood sample.
Some dentists also collect the blood themselves. In Berlin but also in some other large cities, your patients can have blood samples collected in our partner facilities. We are happy to provide you with more information.
How are the patient samples sent to the laboratory?
The samples are shipped by courier (page 69) or also by post in some cases.
A stamped shipping envelope is included in each LTT blood collection set. The sample must always be sent to the laboratory on the day the blood is collected. The blood must be received by the laboratory within 24 hours.
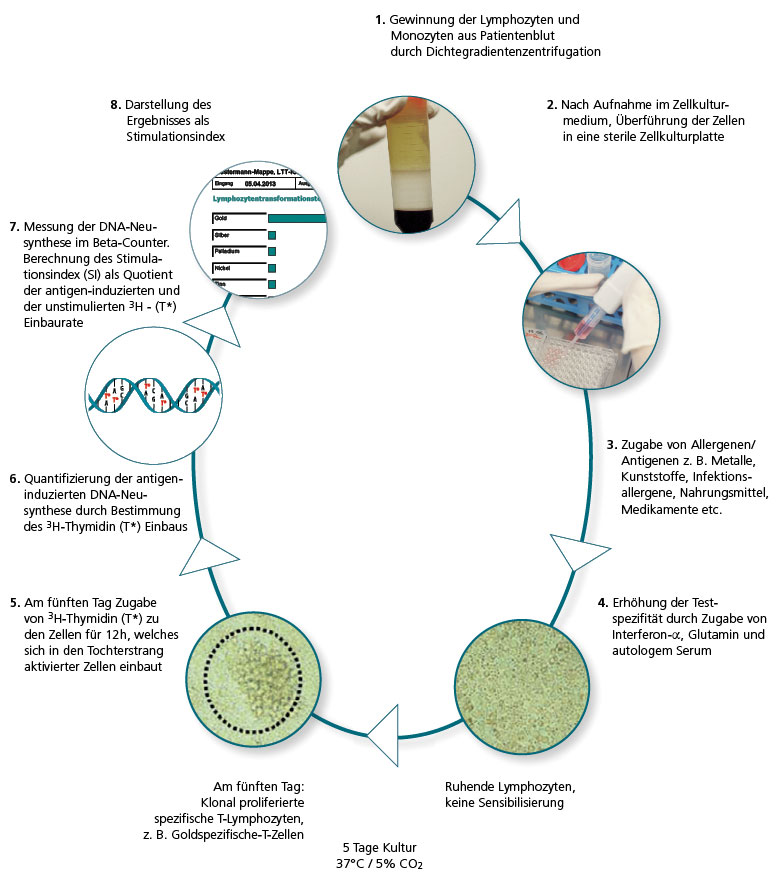
Performing the LTT
In the LTT the lymphocytes are extracted from the patient’s blood sample and then challenged with the allergen being tested in three parallel batches. If there are allergen-specific T lymphocytes present in the patient’s blood, over the next 6 days clonal cell proliferation is seen. This is quantified using DNA synthesis.
The allergen-induced lymphocyte proliferation is compared to the spontaneous proliferation (blank value), which is used to determine the stimulation index (SI).
How does the LTT of today differ from methods used in the past?
Even at the turn of the century, the sensitivity of the LTT was still comparatively low. In type IV allergy testing, the LTT was at best equal to the skin test, if not inferior.
However, the LTT technologies used today in special immunological laboratories are characterised by high sensitivity and specificity.
Refinements in cell culture techniques and media, the purity of the allergens used for the cell stimulation and key improvements made to the techniques used to measure 3H thymidine activity that are now available have all contributed to this change.
The use of genetically engineered interferon-α as an additive in the cell culture also contributed to the refinement of the method (von Baehr et al., J. Immunol. Methods 2001; 251: 63–71). IMD-Berlin started using this optimised LTT version in 2005 and it has significantly improved the sensitivity and specificity, particularly compared to the MELISA® method commonly used at the time.
The LTT is an accredited laboratory method.
The lymphocyte transformation test is a demanding laboratory procedure. Along with expensive laboratory equipment, extensive experience and care are required of the laboratory personnel carrying out the test because only a few of the steps in the test can be automated. Even in today’s age of modern technology, good old manual processing by experienced staff trained in cell culture techniques is still at the heart of cell cultures.
The analytical quality also depends on the degree of standardisation in the particular laboratory and on the methodology that has been established and validated for each individual allergen. For this reason, cell-based analytical methods should only be used by specialized laboratories and institutes focused on these technologies and these methods should be accredited according to DIN 15189 by the National Accreditation Body for the Federal Republic of Germany (DAkkS).
IMD-Berlin has been accredited to use the LTT according to DIN 15189 in 2004 and is regularly monitored.
The LTT is preferable to the epicutaneous test (ECT) for dental issues.
To detect type IV sensitisation, two independent test methods are available: the LTT and the epicutaneous test. For some questions, these complement each other well (e.g. in occupational dermatology). For dental questions, however, the LTT is preferable to the ECT for the following reasons:
1. The LTT is more sensitive for systemic sensitisations.
The epicutaneous test is validated for verifying a contact allergy, that is, for allergies where the sensitisation has taken place via skin contact or has manifested on the skin. For allergens that are taken up via the mucous membranes, these lead to systemic sensitisations. The LTT has advantages here in terms of the sensitivity because it is performed using blood cells.
Because of this, the LTT was included as a test for the detection of sensitisation to medications in the allergologic guidelines and classed as ‘recommended without reservations’ by the Guidelines Committee of the Robert Koch Institute.
Even if professional dermatology associations still question it, allergens from dental restoration materials are taken up via the mucous membranes and not via the external skin barrier, in a similar way to medications.
The logical step to also recommend the LTT in this case failed in the past rather due to the lack of regard for the issue of ‘intolerance of dental restoration material’ rather than due to immunopathological arguments against the LTT.
The sensitivity of the LTT is stated as being 90% to 95% depending on the test allergen. The specificity depends on the validation of each individual allergen and the quality of the implementation of the LTT. In a laboratory accredited according to DIN 15189, false positives due to non-specific activations can be ruled out because of internal standardisation.
2. With the LTT the patient does not come into contact with the allergen.
Due to the unavoidable direct contact, the patient has with the allergen in the epicutaneous test, the patient can be sensitised by the test itself. This is not possible with the LTT because the challenge is done using a blood sample in the laboratory. What is problematic with the epicutaneous test is that a sensitisation that is developing cannot be detected in the initial epicutaneous test.
The sensitisation can only be detected in a second epicutaneous test carried out at least 10–14 days later. This second ‘test’ is carried out paradoxically by the dentist when he or she inserts the substitute material into the body. For this reason, the epicutaneous test is actually contraindicated for preventive testing.
Carcinogenic and toxic substances should never be applied to a patient’s skin, which is why their use is forbidden in the epicutaneous test.
The Methods and Quality Assurance Committee of the Robert Koch Institute summarises the advantages of the LTT as follows:
Quality assurance with the lymphocyte transformation test’ – Addendum to the LTT paper of the RKI Methods and Quality Assurance in Environmental Medicine Committee, notification of the Methods and Quality Assurance in Environmental Medicine Committee, Bundesgesundheitsblatt – Gesundheitsforschung – Gesundheitsschutz 2008; 51:1070–1076 |
Literature