//en/special-areas-of-competence/dentistry/allergies-and-intolerances
Lymphocyte transformation test (LTT)
Can dental restoration materials cause allergies?
Metals and acrylates (plastics) as well as many other components present in root canal filling materials or cements may be potential allergens.
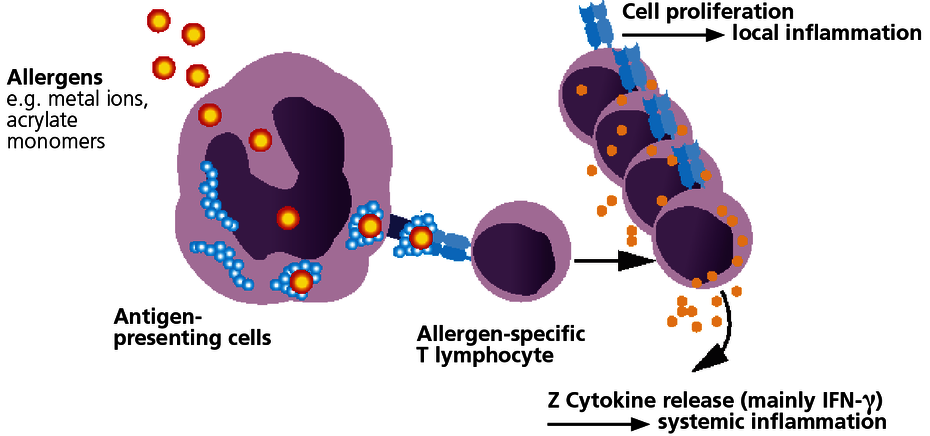
With few exceptions, sensitisation to dental materials is based on type IV sensitisations (late-type allergies). With this type of allergy, specific T lymphocytes form which identify as foreign the allergen or the body’s own protein that has been modified by the allergen (hapten effect). In sensitised patients, the immune system responds to contact with the relevant allergen with immune activation. This can be expressed as local symptoms but may also cause or amplify systemic inflammatory reactions.
Type IV sensitisation to an allergen then exists once the patient has developed allergen-specific T lymphocytes. This is verified with the LTT.
When does intolerance to dental materials caused by an allergy have to be considered?
Local signs include stomatitis, lichen ruber planus, gingivitis or periodontitis. They are not, however, obligatory because the oral mucous membranes are immunologically less reactive. Because of the special immunological characteristics of the oral mucosa, local symptoms such as a burning tongue or jaw pain and toothache are therefore not associated with a morphological correlate.
Because immune reactions have a systemic character as a matter of course, with type IV allergies many generalised symptoms may be present or be increased. These non-specific inflammatory symptoms include exhaustion, sleep disorders, depressed mood, muscle pain, arthralgia (fibromyalgia), paraesthesia, headaches, migraines or even neuralgia.
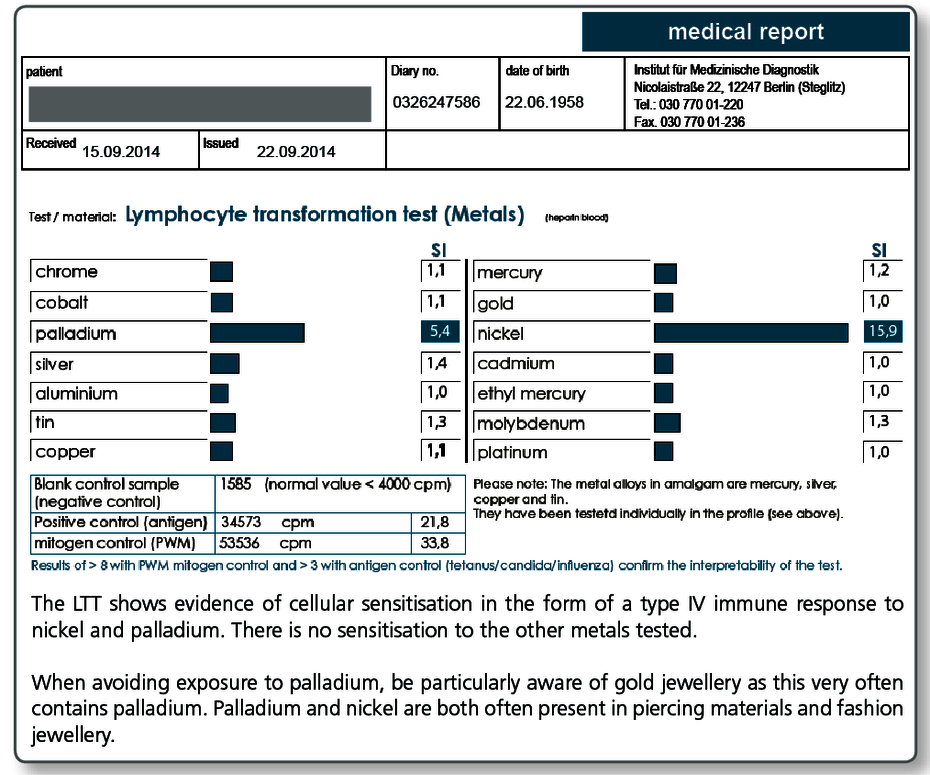
It is also known that prolonged exposure to metal ions (including mercury, gold, nickel) can trigger autoimmunity in sensitised patients.
The LTT can detect allergen-specific T lymphocytes.
The LTT is a laboratory method to detect a specific cellular sensitisation. The test is based on the principle of allergen-specific induction of cell division in lymphocytes after contact with their ‘matching’ allergen. A positive reaction in the LTT indicates the presence of allergen-specific lymphocytes (memory cells) in the patient’s blood.
There are two questions that can be answered with the LTT:
- Is it necessary to replace the existing dental restoration material?
Using the LTT, a correlation to the material can be verified or excluded if symptoms develop after the dental restoration material is applied (curative clinical question). - What materials can be used or should not be used?
Using the LTT, existing sensitisations to any of the components of a new dental restoration material can be excluded prior to its use (preventive clinical question).
Which materials can be tested for using the LTT?
In principle, almost all dental restoration materials can be tested in the LTT provided a cytotoxic effect or non-specific activating factors can be excluded in the laboratory.
For frequently occurring clinical questions, the following profiles have been developed in which the known individual sensitising allergens undergo standardised testing.
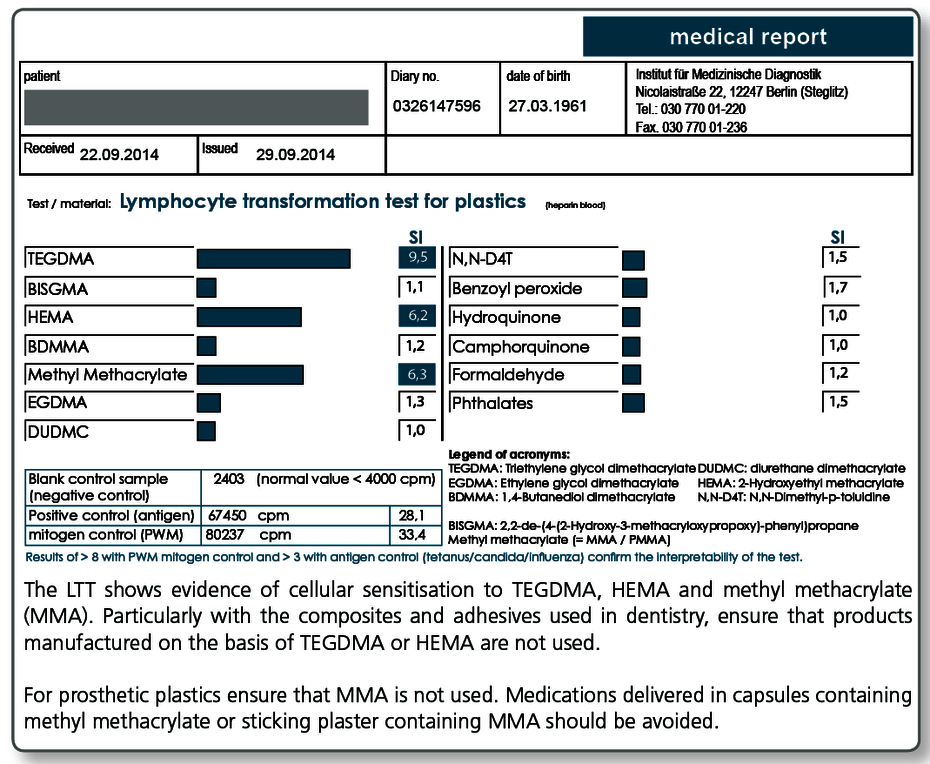
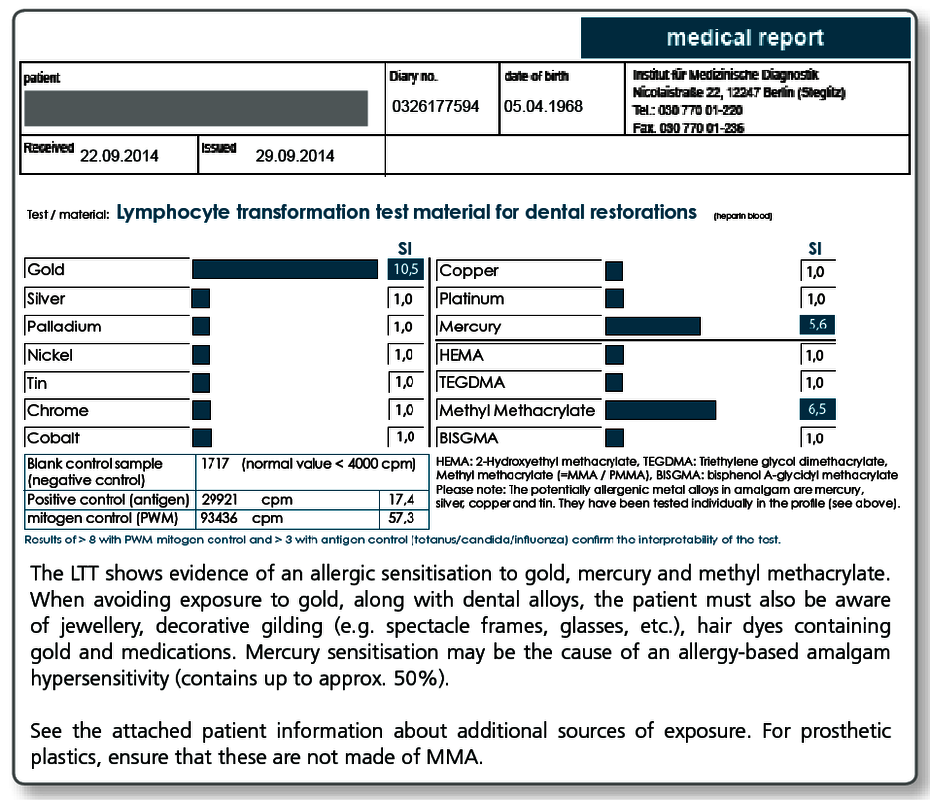
| LTT Metals | Gold, nickel, palladium, chromium, cobalt, molybdenum, aluminium, platinum, cadmium, mercury, copper, silver, tin, ethylmercury |
| LTT Plastics | Methyl methacrylate (MMA/PMMA), TEGDMA, BISGMA, BISDMA, HEMA, diurethane dimethacrylate, ethylene glycol dimethacrylate, butanediol-1-4-methacrylate, N,N-dimethyl-4-toluidine, benzoyl peroxide, hydroquinone, camphorquinone, phthalates, formaldehyde |
| LTT Combination Profile (Dentalcheck) | Gold, nickel, palladium, chromium, cobalt, platinum, mercury, copper, silver, tin, methyl methacrylate (MMA/PMMA), HEMA, TEGDMA, BISGMA |
| LTT Gold Alloys | Gold, silver, platinum, copper, palladium, tin, gallium, indium, iridium, rhodium, tantalum, ruthenium |
| LTT Amalgam | Amalgam components and organic mercury compounds: mercury, copper, silver, tin (amalgam), ethylmercury, phenylmercury, methylmercury |
| LTT Root Canal Filling Materials | Raw gutta-percha, balsam of Peru, eugenol, polydimethylsiloxane, silicone oil, bismuth oxide, silver, turpentine oil, colophonium, triethanolamine, peanut oil, paraformaldehyde, bisphenol A, epichlorhydrin |
| LTT Ceramic + Cements | Vanadium, aluminium, titanium, cobalt, chromium, barium, silicon, cerium, boron, manganese, antimony, phosphate cement (Harvard), glass ionomer cement (Ketac-Bond) |
| LTT Native Materials | Testing of a range of selected materials that can also be sent to us in the laboratory. |
Please refer to the detailed description regarding the diagnosis of titanium intolerance.
Are there cost-effective combination profiles?
A combination profile (Dentalcheck) was developed specifically for preventive testing which includes the most important metals and acrylates and covers the following problematic areas:
- Gold alloys (gold, silver, palladium, platinum, copper and tin)
- NPM alloys (chromium, cobalt, nickel)
- Amalgam (mercury, silver, copper, tin)
- Prosthetic plastics (MMA/PMMA)
- Composites and cements containing acrylates (HEMA, TEGDMA, BISGMA)
Can native and custom materials also be tested?
Yes, if the materials are sent to the laboratory along with the blood sample. This procedure has proven itself particularly useful for complex materials such as cements, composites, prosthesis materials, bone graft substitute materials, root canal filling materials (also points) as well as intraoral harvested metal and plastic chips and shavings.
Negative test results for the materials exclude sensitisation to any of the components (declared or not declared). Materials that test positive in the LTT must not be used under any circumstances because there is a type IV sensitisation to at least one of the allergens present.
Note: Native materials frequently used in dentistry are kept in stock in the laboratory. Please call us under +49 30 77001-220 and we will send you this list per fax.
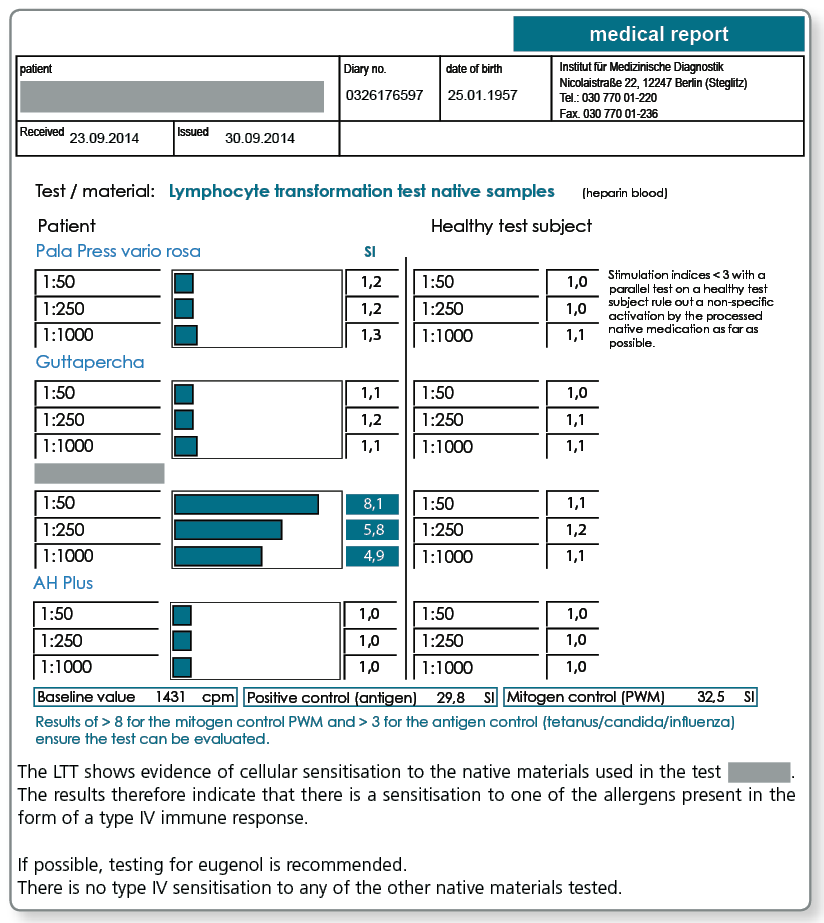
LTT Root Canal Filling Material
There may be allergic sensitisations to gutta-percha but also to other components present in sealers. The known allergens are included in the LTT Root Canal Filling Material profile.
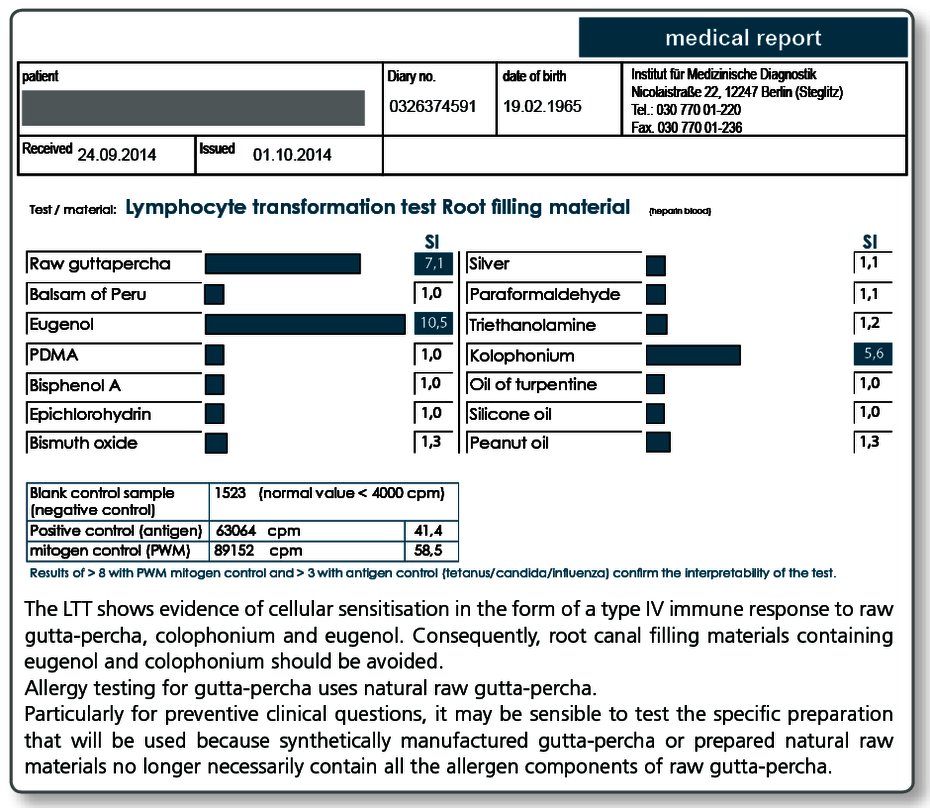
Allergy testing for gutta-percha uses natural raw gutta-percha. Particularly with preventive queries it can be useful to test the specific gutta-percha preparation (points as well) to be used as native material in the LTT, because, for example, synthetically produced gutta-percha or processed natural raw materials no longer necessarily contain all the allergen components present in raw gutta-percha.
To illustrate the importance of the components included in the LTT profile, the (known) important components of commonly used temporary or permanent root canal filling materials are listed below.
The ingredients were taken from the material safety data sheets or the product information. We do not assume any liability for the correctness and completeness of this list.
| N2 Endodontic Cement® | ingredients include paraformaldehyde, titanium oxide, zirconium oxide (powder) and eugenol, rose oil, lavender oil, peanut oil (liquid) |
| Endomethasone® | ingredients include thymol iodide, zinc oxide, hydrocortisone acetate (powder) and eugenol (liquid) |
| AH Plus® | bisphenol A diglycidyl ether, bis [4-(2,3-epoxypropoxy)phenyl]-methane, PDMS |
| AH26® | ingredients include bismuth oxide, methenamine, titanium oxide, silver |
| Aptal-Harz Wurzelfüllung® | ingredients include zinc oxide, colophonium (powder), eugenol, balsam of Peru, turpentine oil (liquid) |
| Rocanal Permanent Vital R2® | ingredients include aniseed oil, phenylphenol (powder), eugenol, colophonium, castor oil (liquid) |
| Hermetic® | ingredients include zinc oxide, zinc stearate, zirconium(IV) oxide (powder), eugenol, balsam of Peru (liquid) |
| Super EBA® | ingredients include eugenol (liquid), zinc oxide, aluminium oxide (powder) |
| Apexit Plus® | ingredients include calcium hydroxide, calcium oxide, colophonium |
| Gutta-percha points | depending on the brand, contain zinc oxide, purified gutta-percha, colophonium (1–4.1%), heavy metals 1.5–30%, possibly pigments, food dyes |
What are the consequences of a positive LTT result?
A positive result in the LTT indicates that there is a type IV sensitisation to the allergen concerned. Materials that contain this allergen should not be used in future (preventive statement). For curative issues, ensure that neither an allergy test (nor the epicutaneous test) is used to verify the direct causal relationship with the existing symptoms.
With a verified sensitisation, it must be carefully weighed up whether the particular problem material should be removed and replaced. What is critical here is the clinical symptoms rather than just a positive test result. Other sources of exposure must be primarily or simultaneously eliminated (detailed information about other sources of exposure can be found in the LTT results).
In an individual case with verified sensitisation and unclear clinical significance, the causal relationship between the sensitisation and the clinical manifestation can be examined in more detail by subsequently carrying out effector cell typing.
What do you need for the LTT?
For each examination profile, 20 mL heparin and 5 mL whole blood are required for serum extraction.
The collection and shipping material is provided by the laboratory free of charge. The blood collection sets are available from all the usual manufacturers.
Please contact our staff in the regional practice support team or our service personnel in the laboratory and they will happily answer all your questions about organising the LTT.
How is the blood collected?
Normally dentists send their patients to their general practitioner with the blood collection set and the completed request form, and he or she then collects the blood sample.
Some dentists also collect the blood themselves. In Berlin but also in some other large cities, your patients can have blood samples collected in our partner facilities. We are happy to provide you with more information.
How are the patient samples sent to the laboratory?
The samples are shipped by courier (page 69) or also by post in some cases.
A stamped shipping envelope is included in each LTT blood collection set. The sample must always be sent to the laboratory on the day the blood is collected. The blood must be received by the laboratory within 24 hours.
Performing the LTT
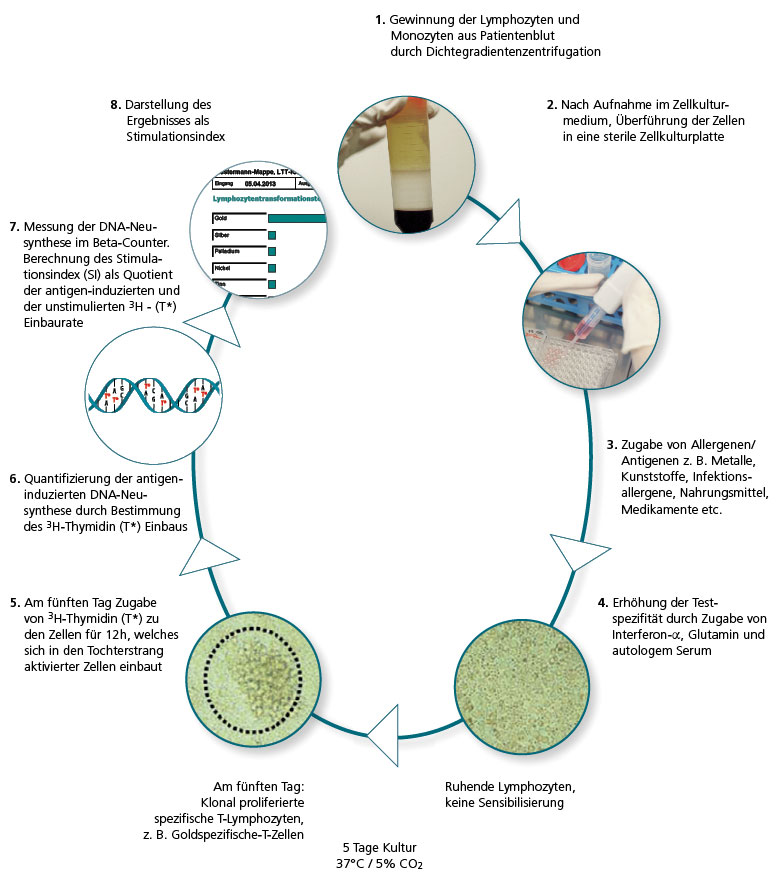
In the LTT the lymphocytes are extracted from the patient’s blood sample and then challenged with the allergen being tested in three parallel batches. If there are allergen-specific T lymphocytes present in the patient’s blood, over the next 6 days clonal cell proliferation is seen. This is quantified using DNA synthesis.
The allergen-induced lymphocyte proliferation is compared to the spontaneous proliferation (blank value), which is used to determine the stimulation index (SI).
How does the LTT of today differ from methods used in the past?
Even at the turn of the century, the sensitivity of the LTT was still comparatively low. In type IV allergy testing, the LTT was at best equal to the skin test, if not inferior.
However, the LTT technologies used today in special immunological laboratories are characterised by high sensitivity and specificity.
Refinements in cell culture techniques and media, the purity of the allergens used for the cell stimulation and key improvements made to the techniques used to measure 3H thymidine activity that are now available have all contributed to this change.
The use of genetically engineered interferon-α as an additive in the cell culture also contributed to the refinement of the method (von Baehr et al., J. Immunol. Methods 2001; 251: 63–71). IMD-Berlin started using this optimised LTT version in 2005 and it has significantly improved the sensitivity and specificity, particularly compared to the MELISA® method commonly used at the time.
The LTT is an accredited laboratory method.
The lymphocyte transformation test is a demanding laboratory procedure. Along with expensive laboratory equipment, extensive experience and care are required of the laboratory personnel carrying out the test because only a few of the steps in the test can be automated. Even in today’s age of modern technology, good old manual processing by experienced staff trained in cell culture techniques is still at the heart of cell cultures.
The analytical quality also depends on the degree of standardisation in the particular laboratory and on the methodology that has been established and validated for each individual allergen. For this reason, cell-based analytical methods should only be used by specialized laboratories and institutes focused on these technologies and these methods should be accredited according to DIN 15189 by the National Accreditation Body for the Federal Republic of Germany (DAkkS).
IMD-Berlin has been accredited to use the LTT according to DIN 15189 in 2004 and is regularly monitored.
The LTT is preferable to the epicutaneous test (ECT) for dental issues.
To detect type IV sensitisation, two independent test methods are available: the LTT and the epicutaneous test. For some questions, these complement each other well (e.g. in occupational dermatology). For dental questions, however, the LTT is preferable to the ECT for the following reasons:
1. The LTT is more sensitive for systemic sensitisations.
The epicutaneous test is validated for verifying a contact allergy, that is, for allergies where the sensitisation has taken place via skin contact or has manifested on the skin. For allergens that are taken up via the mucous membranes, these lead to systemic sensitisations. The LTT has advantages here in terms of the sensitivity because it is performed using blood cells.
Because of this, the LTT was included as a test for the detection of sensitisation to medications in the allergologic guidelines and classed as ‘recommended without reservations’ by the Guidelines Committee of the Robert Koch Institute.
Even if professional dermatology associations still question it, allergens from dental restoration materials are taken up via the mucous membranes and not via the external skin barrier, in a similar way to medications.
The logical step to also recommend the LTT in this case failed in the past rather due to the lack of regard for the issue of ‘intolerance of dental restoration material’ rather than due to immunopathological arguments against the LTT.
The sensitivity of the LTT is stated as being 90% to 95% depending on the test allergen. The specificity depends on the validation of each individual allergen and the quality of the implementation of the LTT. In a laboratory accredited according to DIN 15189, false positives due to non-specific activations can be ruled out because of internal standardisation.
2. With the LTT the patient does not come into contact with the allergen.
Due to the unavoidable direct contact, the patient has with the allergen in the epicutaneous test, the patient can be sensitised by the test itself. This is not possible with the LTT because the challenge is done using a blood sample in the laboratory. What is problematic with the epicutaneous test is that a sensitisation that is developing cannot be detected in the initial epicutaneous test.
The sensitisation can only be detected in a second epicutaneous test carried out at least 10–14 days later. This second ‘test’ is carried out paradoxically by the dentist when he or she inserts the substitute material into the body. For this reason, the epicutaneous test is actually contraindicated for preventive testing.
Carcinogenic and toxic substances should never be applied to a patient’s skin, which is why their use is forbidden in the epicutaneous test.
The Methods and Quality Assurance Committee of the Robert Koch Institute summarises the advantages of the LTT as follows:
Quality assurance with the lymphocyte transformation test’ – Addendum to the LTT paper of the RKI Methods and Quality Assurance in Environmental Medicine Committee, notification of the Methods and Quality Assurance in Environmental Medicine Committee, Bundesgesundheitsblatt – Gesundheitsforschung – Gesundheitsschutz 2008; 51:1070–1076 |
Literature
J Lab Meb 2006;30(2):101-106 © 2006 by Walter de Gruyter - Berlin - New York. DOI 10.1515/JLM.2006.014
Significance of the patch test and the lymphocyte transformation test in the diagnostic of type IV-sensitization.
Statement of the German professional association for enviromental medicine
Frank Bartram1, Hans-Peter Donate2, Kurt E. Müller3, Claus-Hermann Bückendorf4,
Peter Ohnsorge5, Wolfgang Huber6, Volker von Baehr7
1 Praxis für Allgemeinmedizin/Umweltmedizin, Augustinergasse 8, 91781 Weissenburg
2 Praxis für Allgemeinmedizin/Umweltmedizin, Saarbrücker Strasse 9, 66679 Losheim am See
3 Praxis für Dermatologie/ Umweltmedizin, Scherrwiesenweg 16, 88316 Isny
4 Praxis für Allgemeinmedizin/Umweltmedizin, Wolfsbrook 2, 24113 Kiel
5 Praxis für Hals-Nasen-Ohrenheilkunde/Umweltmedizin, Juliuspromenade 54, 97070 Würzburg
6 Praxis für Innere Medizin/Umweltmedizin, Maaßstraße 28, 69123 Heidelberg-Wieblingen
7 Institut für Medizinische Diagnostik, Abteilung Immunologie, Nicolaistrasse 22, 12247 Berlin
Summary
The epicutaneous test is the most commonly used method for the detection of type IV sensitisation to allergens and haptens.
This test is in the hands of experienced investigators mostly very helpful for the evaluation of the role of allergens in contact allergies. However, standardised in-vitro methods are also required especially for the identification of potentially sensitising toxic or carcinogenic substances.
Recent developments in cell culture technology have led to the establishment of modern cellular techniques carried out in specialised laboratories particularly in environmentally-challenged patients as a powerful alternative for the assessment of type IV sensitisation. At the same time as considering the potential of such in-vitro assays one should bear in mind their limitations too.
The lymphocyte transformation test (LTT) represents nowadays an important alternative for the detection of a specific type IV sensitisation. Advantages and disadvantages of both procedures regarding their diagnostic specificity and sensitivity and the arising conclusions for application in routine diagnostics will be discussed. Keywords epicutaneous test, lymphocyte transformation test, type IV sensitisation, allergen, hapten
Epicutaneous test (ECT)
When diagnosing allergic contact eczema (type IV allergy), the epicutaneous test (patch test) is most frequently used. The test principle is based on the fact that allergen-specific T lymphocytes migrate to the patch of skin to which the test allergen has been applied where they induce a macroscopically apparent skin infiltrate after 24 to 72 hours. When assessing positive test reactions, allergic and irritative skin responses must be differentiated. Many contact allergens are also skin irritants, particularly for patients with sensitive skin. When evaluating an epicutaneous test, to avoid false positives and false negatives the examiner must consider the varying penetrative ability and immunological responsivity of different skin types as well as the different sensitivity and reproducibility of the individual test allergens. For these reasons, epicutaneous testing should remain the preserve of examiners who are well trained in allergology and who are familiar with the limits of this established routine test.
The lymphocyte transformation test (LTT)
The LTT is currently the only comprehensively validated in vitro method for detecting specific cellular sensitisations. It is based on the principle of antigen (allergen) induced cell division of specific T lymphocytes and analysis of the induced DNA synthesis. A positive reaction in the LTT verifies the presence of allergen-specific T memory cells in the patient’s blood. The optimised LTT versions in use since 2002 have achieved increased sensitivity and specificity thanks to the addition of recombinant interferon-alpha to the lymphocyte culture (1, 2, 3).
Until a few years ago, the LTT had reduced sensitivity but was still equivalent to the skin prick test. The specificity was limited due to borderline reactions that were not uncommon. The diversity in the methods used and the lack of standardisation at the time explain the very different evaluations of the LTT prior to 2000. Along with studies that attested to the high sensitivity and specificity of the LTT as well as its clinical relevance even then (4, 5, 6), there are also publications reporting on false positive results (7, 8).
Over the last 5 years, the importance of the LTT as well as the body of data on the sensitivity and specificity has changed significantly. Further developments in cell culture techniques, the quality of the antigens used and not least improved measurement methods have all contributed to this. The liquid scintillation devices previously used for tritium counting have been replaced by highly sensitive, automated solid-phase b counters. As a result of these methodological developments, the LTT was accredited in its current standardised form as a test procedure by expert reviewers from the Deutsche Akkreditierungsstelle Chemie GmbH (German accreditation body for chemistry) in spring 2003 according to DIN EN 17025 and since January 2005 according to DIN EN 15189.
‘Non-specific mitogenic effects’ can be excluded in the LTT
In the older technical literature, there are articles (no studies) that postulate that non-specific mitogenic effects occur with the LTT when testing for metals. This can be reliably excluded by the validated, standardised concentrations of the allergens used. These are also not ‘mitogenic effects’ because the cells that proliferate in the LTT are exclusively CD4 T helper cells. Positive LTT reactions without clinical contact allergies are based on balanced sensitisations with an immunological tolerance is maintained by CD25+ regulatory T cells (9). IL-10-secreting CD4 cells are also involved (10). The postulated ‘non-specific activations’ in the LTT must also not be confused with the toxic irritative inflammatory reactions that occur in the skin prick tests because non-specific effects in the LTT can be excluded by using standardised test concentrations and parallel antigen inhibition tests (11).
Sensitivity and reproducibility of the epicutaneous test
Despite great advances in the standardisation of the test allergens, the ‘weak points’ of epicutaneous testing are the subjective test evaluation and the varying nature of the skin of the test persons. The opinion still frequently voiced these days, that epicutaneous testing is in principle superior to the LTT in its predictive ability, must be critically examined.
Several clinical trials show that the sensitivity of epicutaneous testing is too low for a ‘gold standard’. Negative epicutaneous tests with existing clinically verified sensitisation have been repeatedly described (12, 13, 14). Rustemeyer showed for nickel that of 74 patients with clinically verified nickel sensitisation, only 40 had a positive reaction of the skin, which corresponds to a sensitivity of only 54% (10). Bourke showed that epicutaneous testing with various contact allergens, each carried out in duplicate on both sides of the spine on the patient’s back, had an intra-assay reproducibility of about 92% (15). Multicentre studies yielded figures of 84% (16) or only 56% (17). In the study conducted by Gollhausen at the Dermatology Clinic at the Ludwig Maximilians University Munich, patients were examined twice at intervals of one week. In a review published by Iris Ale in 2004, the ratio of non-reproducible reactions from nine studies examined varied from 4.2% to 43.8% (18). It is highly probable that the ‘deviations’ determined in these controlled trials are even higher for routine clinical diagnostics.
Use and scientific reputation of the lymphocyte transformation test (LTT)
The LTT has proven to be equivalent to epicutaneous testing in diagnosing immunologically based medicinal product reactions and superior in its sensitivity (19, 20, 11). This resulted in the positive evaluation by the Robert Koch Institute for this issue (21). However, in the same expert statement the evidence for allergic reactions to environmental pollutants(including metals) was assessed critically ‘because there is no adequate test material available’. That a high sensitivity is ascribed to the identical test for haptens such as derivatives of medicinal products but not for others such as metals is incomprehensible. Precisely for metals such as chrome, nickel and cobalt, more recent studies show that the LTT has a higher sensitivity compared to epicutaneous testing (22, 23).
In the expert opinion from the RKI mentioned, it is also postulated that positive LTT results ‘only indicate exposure’ that does not always have to be linked to clinical symptoms. A sensitisation verified in the LTT (as well as in the skin prick test!) does not actually have to be associated with clinical symptoms but it does not cast doubt on the sensitisation that is present. It is known that not every sensitisation leads to an allergy. That positive LTT results ‘only indicate exposure’ is also not conclusive because in this case the rate of positive reactions to dental metals, nickel or even cadmium (present in cigarette smoke) is undoubtedly far higher than would have to be demonstrated. The prevalence of positive responses in the LTT is far below the high number of people with relevant exposure (4, 22).
To detect a type IV sensitisation to beryllium, the LTT is undisputedly the tool of choice (24, 21). There is no conclusive explanation of why this is the case for beryllium but not other metals. Metals are particularly suitable for LTT testing because, unlike medications, they are not metabolised. At the Institute for Clinical Immunology of the University Hospital Essen, the correlation of the various test methods LTT, epicutaneous testing and cytokine analyses to each other and to the clinical results was recently examined (25). There was a good correlation of the results of the LTT, the epicutaneous test and the cytokine analyses. Compared to the clinical picture, both the LTT and the epicutaneous test correlated (epicutaneous test, r=0.73, p<0.0001; LTT r=0.74, p<0.0001).
For intolerances to perfume (26), iodine-containing contrast agent (27) and methacrylates (28), it has been demonstrated that the LTT is useful for the often problematic differentiation between allergic and irritative reactions.
All recent studies show that the validity of the LTT results determined is more dependent on the quality of the test implementation than on the methodology itself. This is no different to other laboratory methods, however. The undifferentiated scepticism found in older publications regarding the LTT is no longer justified in light of the methodological developments and recent studies.
Sensitisation does not have to be associated in every case with local symptoms at the contact site.
Particularly in dental clinics, it is often assumed that a clinically relevant sensitisation absolutely must be associated with oral symptoms. This is not the case because the hapten presentation of the oral mucous membrane and the epidermis differ due to particular immunological features (29, 13). The reason lies in the different lipid composition of the mucous membranes and epidermis (30), more rapid removal of allergens due to the greater blood flow of the reticular layer (31) and the primary immune response influenced by about 400 bacterial species in the oral cavity (32). The Langerhans cells primarily responsible for the initial local inflammatory reaction have functional differences between the epidermis and the mucous membrane. Langerhans cells in the mucous membrane express greater amounts of Fc epsilon RI receptor (33, 34) and are able to induce an allogeneic T cell stimulation in vitro (35). Comparative provocation tests showed that the allergen concentrations required to trigger a mucous membrane response are 5 to 12 times higher than those required for the skin (36).
To develop an allergic mucous membrane response to a foreign substance, it is also critical that the primary contact, that is, the formative immunological recognition process, takes place via the mucous membrane, the skin or the intestine. Both in the mouse model and in humans, the reactivity of the oral mucous membrane can be suppressed by previous intestinal allergen exposure (37, 38).
The differences cited explain why positive epicutaneous test reactions are not always associated with oral manifestations but that at the same time contact allergic mucous membrane responses are not always apparent in every case in epicutaneous testing (16).
LTT and epicutaneous testing can only demonstrate sensitisation but not an allergy.
Evidence of an immunological sensitisation, such as that produced by the LTT and the skin prick test, is not obligatorily associated with clinical local or system symptoms. An allergy diagnosis can only be made in light of the clinical results and the medical history. Allergy tests support the diagnosis because sensitisation is an essential prerequisite for an allergy. In our opinion, epicutaneous testing and an LTT carried out in a cell culture laboratory specialising in the procedure are two methods that complement one another well in difficult cases.
Our experience over the previous four years shows that an LTT carried out in a standardised manner is important particularly for the following issues:
- Negative epicutaneous test result with urgent clinical suspicion of a contact allergy
- Dubious positive results in the epicutaneous test (toxic reactions?)
- Preventive testing before using dental restoration material or queries from professional organisations
- Testing of potentially sensitising or carcinogenic substances
For preventive queries, the LTT should be used.
In principle, for preventive queries regarding existing type IV sensitisations, such as those carried out primarily in dentistry, epicutaneous testing should be used with restraint becausethe application of the test substance to the skin presents a potential risk of sensitisation (39, 40). Agrup showed in one study in which epicutaneous tests were carried out twice for the standard allergens that with repeated testing there was a significant number of new sensitisations after 6 months. The prevalence of iatrogenic sensitisation is 5% for cobalt, 4.6% for p-phenylene diamine, 2.3% for chrome and 9.9% for p-aminoazobenzene, for example (39). Additional case descriptions are available for benzoyl peroxide, butyl hydroquinone, composite mix (41), 4-tert-butylcatechol (42), various plant extracts (43), budesonide (44), formaldehyde (45), nickel (10) and acrylates (46). For penicillin, therapeutic cutaneous application is still considered malpractice due to the risk of sensitisation (41). In animal trials, sensitisation to chrome, mercury and nickel can be induced, for example (47). In light of this, the common practice of routine epicutaneous testing of the complete standard series of allergens must be critically evaluated.
The procedure of confirming a positive LTT result using an epicutaneous test should therefore only be done in exceptional cases, e.g. with borderline results, because amplification of the clinical symptoms has been demonstrated for various contact allergens as a result of exposure to the test contact allergen (48, 49, 50). Anyway, the limited sensitivity and lack of reproducibility (12, 13) restrict the indications for such ‘follow-up tests’.
From the standpoint of curative environmental medicine, to evaluate positive test results, the following take-away messages are recommended:
- For preventive examinations with potentially sensitising substances, all tests with carcinogenic materials and the testing of patients with predisposing immunological disorders, the LTT as a non-stressful procedure should be preferred to epicutaneous testing.
- A reliably positive result in the epicutaneous test ensures sensitisation. Dubious positive results and results suspected of being due to toxic responses in epicutaneous testing should be confirmed by the LTT even for curative cases.
The quality standards must be high for cellular laboratory procedures!
The use of the LTT in clinical diagnostics places stringent requirements on the treating doctor in terms of the indication and on the laboratory carrying out the test to ensure consistently high quality for the test implementation. The LTT is reserved for specific issues due to the high costs compared to epicutaneous testing. Considering the literature described here, it would be wrong, however, to not implement this modern diagnostic in vitro method.
However, what must be stipulated is that this demanding cell culture method is performed exclusively by accredited medical institutes which have sufficient experience using the method. The mandatory ring and comparative tests with test laboratories prescribed for laboratories accredited according to DIN ISO 15189 are a critical prerequisite for effective quality management and reliable consideration of the test results in clinical practice.
Important note: Crucial parts of the RKI statement mentioned in the text were corrected in 2008. The statement from the German Professional Association of Environmental Medicine was nevertheless printed here in the original form from 2006 so as not to violate copyright. |
Literature
Titanium intolerance
Titanium is characterised by outstanding corrosion properties, meaning that compared to other metals it is well tolerated immunologically. However, it is known that for some patients implants induce adverse signs of inflammation that may lead to a lack of osseous integration, gingivitis and peri-implantitis.
True cellular type IV allergies to titanium are rare compared to other metals. The reason is that titanium ions have a high affinity for oxygen and so form oxides immediately after their release and, unlike free ions, these oxides cannot form protein bonds and thus allergenic (haptenic) effects cannot develop. The frequently heard statement that ‘there are no allergies to titanium’ is very likely correct from a strictly immunological perspective. However, allergies are not the only cause of immunologically related intolerances. The most common cause of individual hypersensitivity to titanium is excessive pro-inflammatory reactivity of the tissue macrophages.
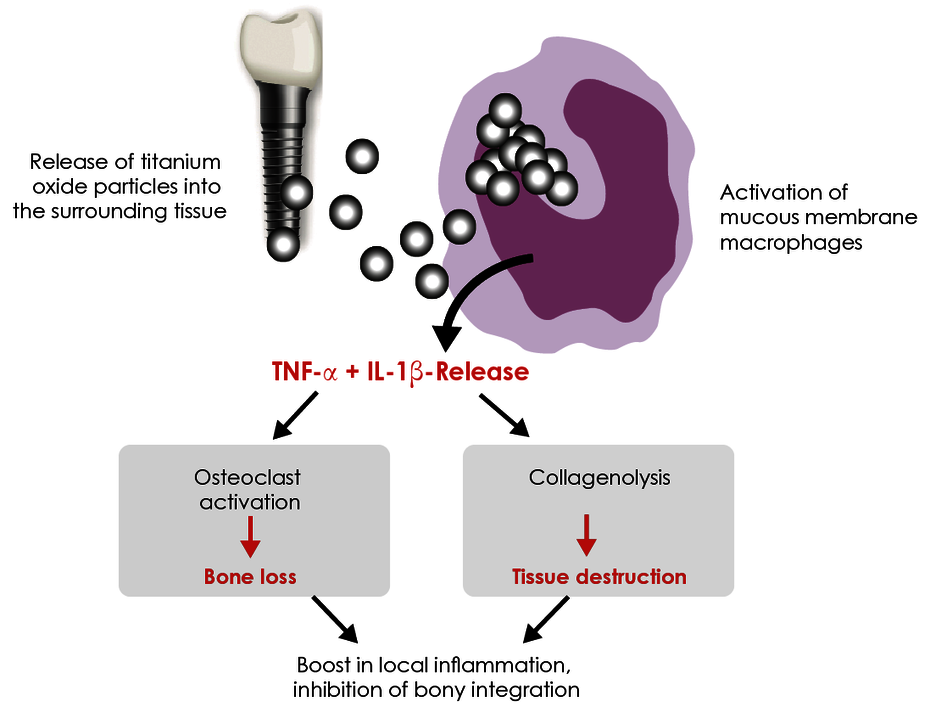
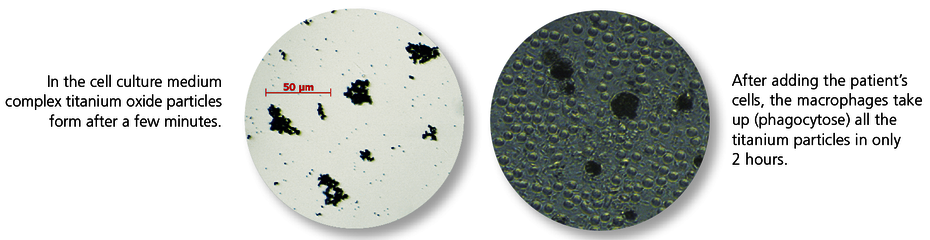
Titanium particles in the tissue induce an inflammatory response.
Metal wear occurs on the surface of implanted titanium materials. Titanium oxide particles are almost always found in the bone and soft tissue surrounding an implant. The tissue macrophages (‘cleaning cells’) phagocytose these titanium oxide particles (particulate debris). It is a physiological response that macrophages react to contact with titanium oxide particles by releasing pro-inflammatory cytokines, in particular TNF-α and interleukin-1. What is highly individual, however, is the extent of this immune response. The intensity of the cytokine release depends on genetic variants (polymorphisms) for the pro-inflammatory (IL-1 and TNF-α) and anti-inflammatory (IL-1RN) mediators involved. Titanium intolerance is therefore usually a consequence of an increased disposition of the tissue macrophages to inflammation in response to titanium oxide particles. Titanium-specific lymphocytes do not play a role here, which explains the negative LTT and epicutaneous test results.
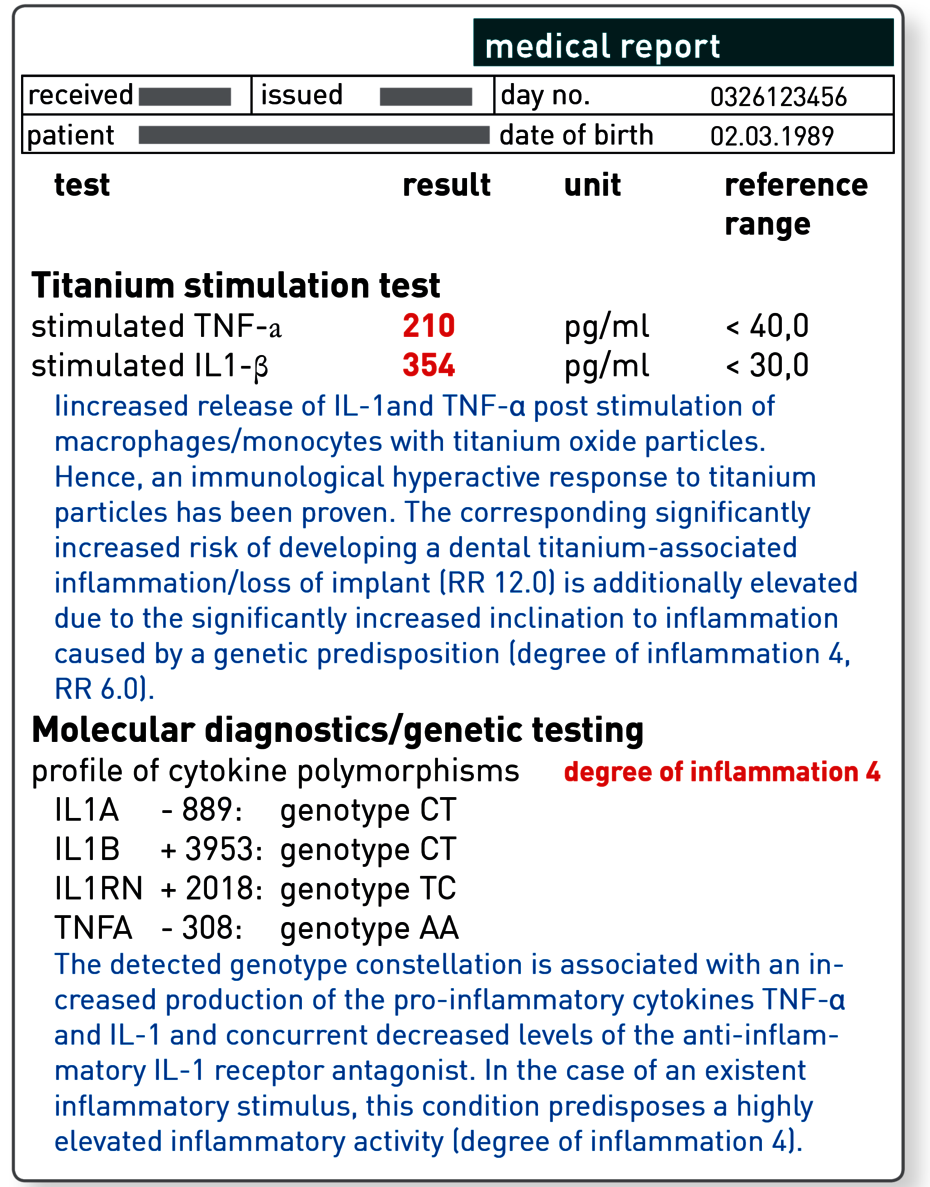
The titanium stimulation test detects the cytokine response of tissue macrophages after contact with titanium oxide.
The titanium stimulation test was developed and comprehensively validated for this issue. This whole-blood stimulation test is used to examine whether the monocytes/macrophages of the patient react to contact with titanium particles with an increased inflammatory response. This is apparent in an increased release of the two key pro-inflammatory cytokines TNF-α and/or IL-1ß. For patients with positive results, delayed or impaired healing of titanium dental implants can be explained by the fact that the macrophages around the implant also have a hyperactive response to released titanium particles and primarily induce local, and in some cases systemic, inflammation.
With an increased genetic degree of inflammation, the risk of developing a titanium-associated inflammation also increases.
For functionally relevant polymorphisms in the genes for the cytokines IL-1, IL-1RN and TNF-α, a link with peri-implantitis or loss of implant has now been demonstrated in a number of studies. Approximately 15 to 20% of the population have a genetically determined response with an exceptionally vigorous inflammatory response. The polymorphisms in the genes for TNF-α, IL-1 and IL-1RN which are responsible have been identified in the laboratory.
This molecular genetic approach has the advantage that it is not affected by any existing inflammation or immunosuppressive therapies. Using the combination of polymorphisms, the genetic testing allows a patient to be assigned a degree of inflammation. The genetic degree of inflammation increases depending on the number of polymorphisms present from grade 0 (no polymorphisms present, normal inflammation susceptibility) to grade 4 (all four polymorphisms tested are present, significantly increased inflammation susceptibility). Patients with grade 3 and 4 are considered high responders and thus are at-risk patients for a titanium-associated dental inflammation or loss of implant.
The clinical relevance of these polymorphisms is also assured by the fact that patients with high responder polymorphisms have an increased susceptibility to developing peri-prosthetic bone loss. It is important to emphasise that the genetic polymorphisms are innate predisposition factors which, unlike an allergy, do not require initial contact before they can be detected in a laboratory test. Consequently, both the titanium stimulation test and the genetic analysis can be used preventively, that is, as part of the implantation planning.
The degree of inflammation and a positive titanium stimulation test are significant independent and thus additive risk factors.
One study which was initiated by the German Society for Environmental Dentistry (Deutsche Gesellschaft für Umwelt-ZahnMedizin, DEGUZ) confirmed the prognostic validity of both analyses (Jacobi-Gresser et al. 2012). Compared to the control group (patients whose implants had healed without any complications at least 5 years ago), patients with implant loss with no stress during healing and patients with implant loss after stress had a significantly higher in vitro release of TNF-α and IL-1β induced by titanium oxide in the titanium stimulation test (p < 0.0001). A positive titanium stimulation test is a risk factor independent of age, sex and smoking status and increases the risk of developing a titanium-associated inflammation or loss of implant twelvefold.
The number of risk polymorphisms and the resultant genetic degree of inflammation also had a significant impact on the implant loss (p* = 0.046). With an increasing genetic degree of inflammation, the risk of developing a titanium-associated dental inflammation or loss of implant increases sixfold.
| Relative risk for loss of a titanium dental implant: Negative titanium stimulation test 1.0 Positive titanium stimulation test 12.0 Genetic degree of inflammation 0 1.0 Genetic degree of inflammation 1 1.5 Genetic degree of inflammation 2 2.4 Genetic degree of inflammation 3 3.8 Genetic degree of inflammation 4 6.0 |
Comment: A patient with a positive titanium stimulation test has a 12-fold higher risk compared to the normal population. If he or she also has a genetic degree of inflammation of 4, the risk increases a further sixfold.
What does a positive result in the titanium stimulation test or an increased genetic degree of inflammation mean?
An abnormal result in one of the two tests indicates the presence of a significant predisposition to developing titanium-associated inflammation, which may be linked to primary or secondary implant loss. It is not the same as an allergy which requires that the allergen has to be avoided as a matter of course. A positive titanium stimulation test or a degree of inflammation of 2 to 4 is therefore not an absolute contraindication for a titanium implant.
However, in these cases alternatives (e.g. ceramic implants, removable bridgework, coated titanium implants) should be critically evaluated and prophylactic measures should be ramped up (prophylaxis, use of reduced rotational speeds, avoiding periodontal probes made of titanium, no immediate implants, removal of the sources of infection, smoking cessation, optimal adjustment of other predisposing diseases (diabetes mellitus), no multiple implants, avoiding any immunostimulation for up to 4 weeks after implantation). The circumstances may require immunosuppressive measures at the time the implant is inserted.
Sample materials required
Titanium stimulation test: 10 mL heparin blood. The heparin Monovettes from the LTT collection set can be used. We are also happy to send you individual collection tubes. The laboratory must receive the samples within 24 hours of collection. The blood should be stored and transported at room temperature.
Genetic inflammation susceptibility: 2 mL EDTA blood or 2 buccal swabs. For the genetic test we require a declaration of consent of the patient. Transport to the laboratory is not time critical and can be done by post.
Should the LTT also be done prior to an implantation or in cases of suspected titanium intolerance?
Type IV sensitisations to titanium are, as mentioned earlier, extremely rare, which is due to the strong tendency of titanium to oxidise. Thus the LTT is far less important for dental issues compared to the titanium stimulation test.
In terms of type IV sensitisations, contaminating metals are certainly of greater relevance. Traces of nickel, vanadium or aluminium can be released from older models of titanium implant. For this reason, an LTT screening profile was developed to supplement the titanium stimulation test in which these three metals are tested in addition to titanium.
Sample materials required
LTT Titanium/Aluminium/Nickel/Vanadium: 20 mL heparin blood and 5 mL whole blood (use the LTT collection sets!). The laboratory must receive the samples within 24 hours of collection. The blood should be stored and transported at room temperature.
Evidence of immediate-type allergies
Type I allergies to acrylate and other non-metal materials
Unlike metals for which allergic intolerance reactions can be attributed exclusively to lymphocyte-mediated type IV sensitisations, immune responses to composite materials containing acrylates as well as other non-metallic materials may also be IgE-mediated responses (type I allergic reactions).
Immediate-type allergies should be considered if the symptoms develop a few hours (in rare cases even minutes) after inserting non-metallic dental restoration materials. Local but also systemic oedematous, wheal-like mucous membrane disorders are common with these rarely having a typical local morphology because of the special features of mucous membranes.
In the literature, there are indications that patients with known immediate-type allergies (hay fever, insect toxin and dust mite allergies) are also more often affected by an acrylate allergy, which in principle suggests a principle predisposition to type I allergies.
How is the testing done in the laboratory?
For many classical type I allergens (e.g. tree pollen), the specific IgE can be determined in automated EAST or RAST procedures in almost every laboratory. Acrylates or, for example, the components of root canal filling materials are not yet commercially available. The diagnostics can therefore only be done using cellular test systems.
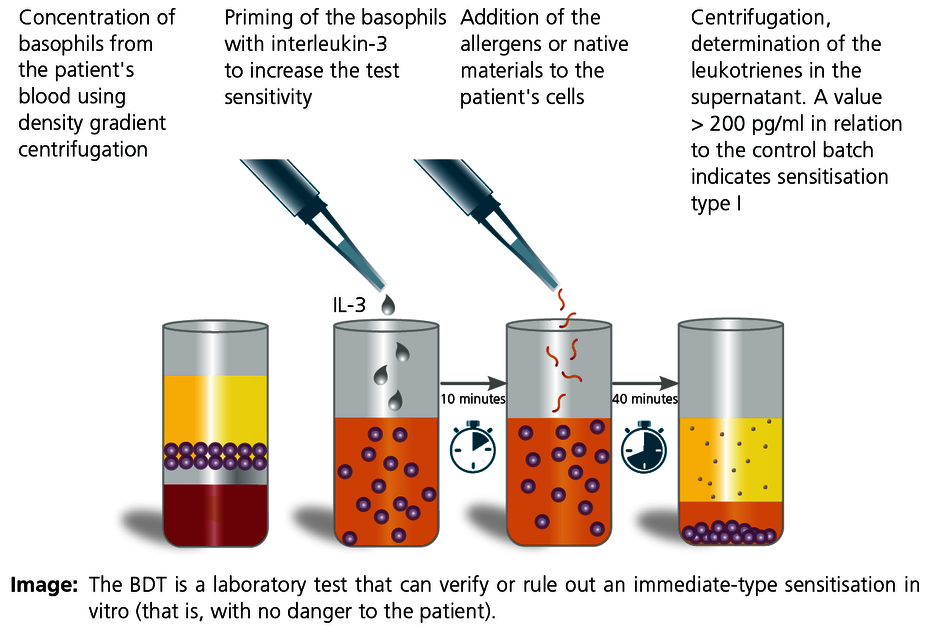
The basophil degranulation test (BDT) is a modern in vitro method to detect type I allergic sensitisation as well as pseudo-allergies. The test is also known as the leukotriene release test, basophil activation test or CAST test. The advantage of this test is that both standard allergens (e.g. acrylate monomers) and native material samples can be tested in the laboratory after preparation. This allows testing of material samples of unknown composition, for example (including from prosthesis material or plastic chips and shavings obtained in situ).
The results of the allergen-stimulated test batches are compared to positive and negative controls. A value greater than 200 pg/mL means that there is a type I sensitisation present. A negative result rules out an immediate-type sensitisation with a high degree of certainty.
Regarding sensitivity and specificity, the BDT has proven to be clearly superior in our laboratory compared to other in vitro provocation tests such as the histamine release test or the CD63 test.
Which dental restoration materials can be tested in the BDT?
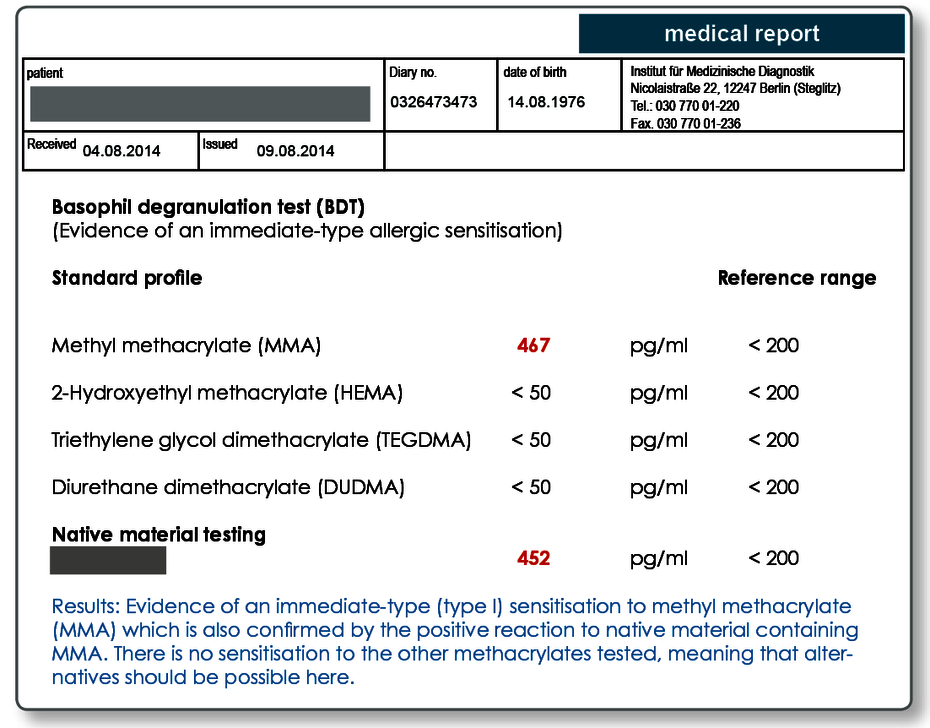
The standard acrylate profile includes:
- Methyl methacrylate (MMA)
- 2-hydroxyethyl methacrylate (HEMA)
- Triethylene glycol dimethacrylate (TEGDMA)
- Diurethane dimethacrylate (DUDMA)
All other common acrylates are available in the laboratory for testing.
Native samples can also be tested in the BDT.
It is also possible to send a material sample (plastic test plates, samples of root canal filling materials, native harvested shavings, cement samples and so on) to the laboratory which can then be directly tested in the BDT. The materials must be sent in together with the blood. A range of materials are also kept in stock in the laboratory and have been established for testing (see the reverse of the dentistry request form). Particularly for preventive testing, the materials should be in the same state as that in which they will later be used in the patient (e.g. cured composite cement, polymerised plastic).
Sample materials required
2 mL EDTA blood or heparin blood for each allergen or native material. The heparin Monovettes in the LTT collection set can be used. We are also happy to send you individual collection tubes. The laboratory must receive the samples within 24 hours of collection. The blood should be stored and transported at room temperature.
Intolerance of local anaesthetic
Hypersensitivity to local anaesthetics may be based on an allergy.
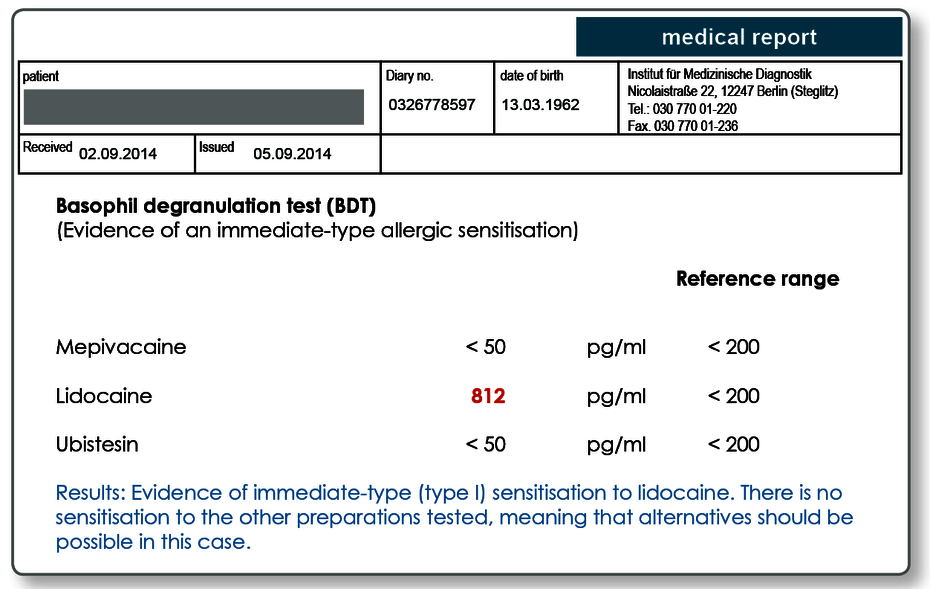
Particularly when symptoms develop within minutes to a few hours, an IgE-mediated sensitisation or pseudo-allergy must be considered a possible cause. Anaphylaxis, that is, systemic hypersensitivity reactions which in some cases may be life threatening have been described. For a suspected intolerance to local anaesthetic, the preparations should be tested using the BDT.
All preparations can be used in the BDT in principle if an ampoule or tablet is sent to the laboratory. The following active ingredients in local anaesthetics are kept in the laboratory and therefore do not have to be sent in: mepivacaine, articaine, lidocaine, prolocaine and ubistesin. For positive results, we prepare an allergy passport. A preparation that has tested positively should not be used as a matter of course.
Sample materials required
2 mL EDTA blood or heparin blood for each preparation. The heparin Monovettes in the LTT collection set can be used. We are also happy to send you individual collection tubes. The laboratory must receive the samples within 24 hours of collection. The blood should be stored and transported at room temperature.
Literature
- Kanerva L. Cross-reactions of multifunctional methacrylates and acrylates. Acta Odontol Scand. 2001;59:320-329.
- Lindström M, Alanko K, Keskinen H, Kanerva L. Dentist's occupational asthma, rhinoconjunctivitis, and allergic contact dermatitis from methacrylates. Allergy. 2002;57:543-545.
- Piirilä P, Hodgson U, Estlander T, Keskinen H, Saalo A, Voutilainen R, Kanerva L. Occupational respiratory hypersensitivity in dental personnel. Int Arch Occup Environ Health. 2002;75:209-216.
- Sanz ML, Gamboa P, de Weck AL. A new combined test with flowcytometric basophil activation and determination of sulfidoleukotrienes is useful for in vitro diagnosis of hypersensitivity to aspirin and other nonsteroidal anti-inflammatory drugs. Int Arch Allergy Immunol. 2005;136:58-72.
- de Weck AL, Sanz ML.Cellular allergen stimulation test (CAST) 2003, a review. J Investig Allergol Clin Immunol. 2004;14:253-273.