Monitoring of immunostimulatory therapies with LTT
The immunocompetence test takes place with the lymphocyte transformation test (LTT).
In addition to the current immunocompetence, the individual response to different
immunostimulatory preparations can be objectivised with the LTT.
CD4 helper cells and cytotoxic CD8 lymphocytes are crucial to the defence against viruses and are involved in tumour surveillance. Functional deficits of these cells can be both the cause and effect of chronic debilitating diseases. The reconstitution of the immune system is a cornerstone of the treatment of reduced resistance to infection and central to the support and aftercare with curative cancer treatments.
Today, the Biological Response Modifiers (BRMs) most commonly used in a medical practice environment are plant lectins (mistletoe products, Echinacea), bacterial lysates (e.g. Arthrokelan®, Gynatren®) and spleen and thymus peptides.
The efficacy in placebo-controlled clinical trials is deemed certain for only some of these substances used for immunostimulation, which stems from the fact that studies of this type for already approved (not patentable) drugs are not really financially viable. It is often overlooked that critics have reported only very limited data demonstrating the lack efficacy of these treatments.
Insights from published case reports about BRMs and numerous studies investigating the mechanisms of action of the products show the following:
- An unspecific stimulation of lymphocytes by BRMs can be scientifically explained and measured in the lab (see Findings Report on the reverse side).
- The number of patients with verifiable therapeutic effect is however only 30% to 60% and varies in terms of the substance class and even the respective preparation.
- Permanent immunostimulation is not generally achieved by adaptation of the immune system through monotonous continuous administration of the same preparation.
- Continued immunostimulation after achieving (for this patient) optimum immune function can result in "flipping” into immunosuppression.
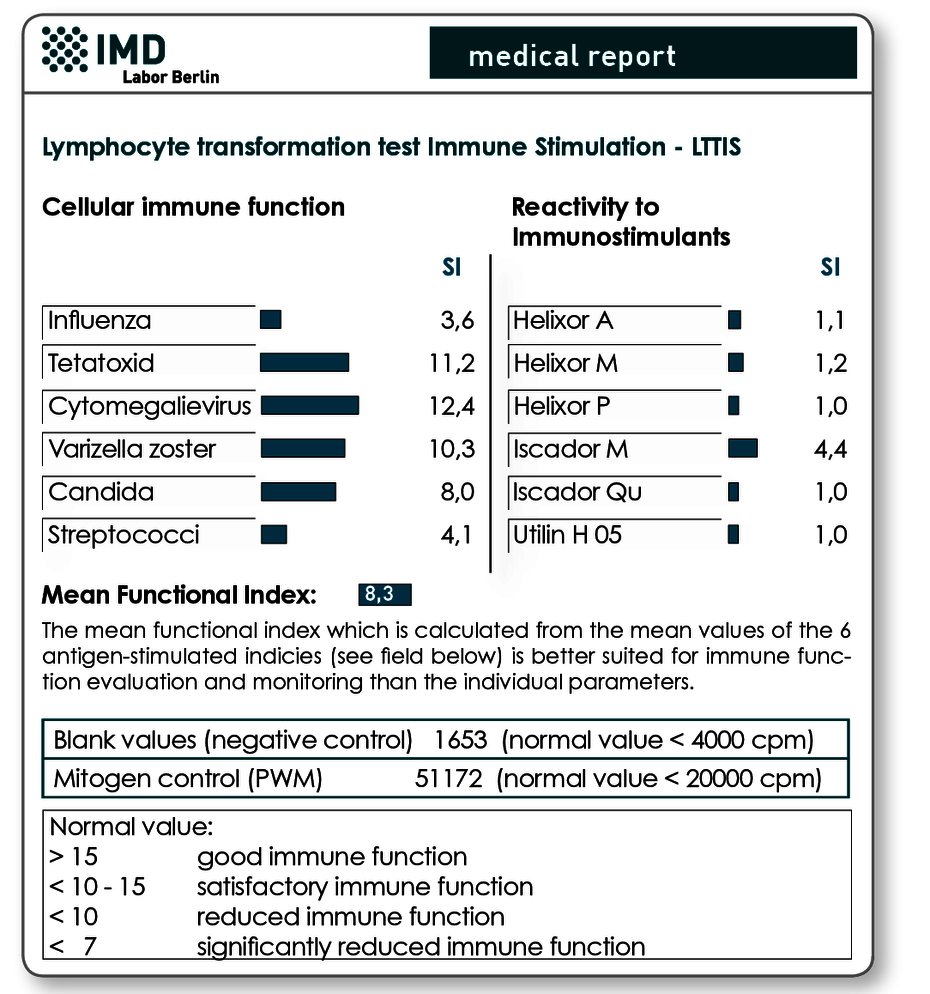
Preliminary findings
Fig. Two results reports are presented for a female patient with metastatic colon cancer before and 8 weeks after beginning an immunostimulatory therapy with Iscador M. The initial finding had shown a reduced T lymphocyte function (left results page) as well as reactivity to Iscador M in the preparation testing. In the control test after 8 weeks, based on the increase in the mean function index from 8.3 to 14.6, it can be seen that the immune function clearly improved with therapy with Iscador M. Successful T cell stimulation with Iscador M is also evident by the significant rise in SI on this preparation. The simultaneous slight increase in SI on Helixor M and Helixor A is due to identical or cross-reactive peptides.
Every immunostimulatory therapy should take place “under visual control”, which means that the target parameter of the therapy, the “immune function”, should be quantitatively logged and checked.
If the therapy fails to have an effect on lymphocyte function (secondary test usually after 4-6 weeks of therapy), the preparation should be exchanged or the application regime changed. The same holds for weakening effectiveness throughout the therapy (follow-up testing recommended quarterly).
The test of immunocompetence in the laboratory takes place today exclusively with the lymphocyte transformation test (LTT)*. Upon request, the individual response to various immunostimulatory preparations is measurable at the same time with this test, since its immune-activating effect is based on a developing T-cell reactivity. (See graphic - Sample findings and “Notes on lab requests”.)
Please note: In contrast to this qualitative method, the cellular immune status which is based on a quantitative analysis of CD4-CD8 and NK cells is not a functional parameter and thus of only very limited use for monitoring immunostimulatory treatments. This test can at best show long-term immunocompetence improvements.
Information from the test |
|---|
Initial test
|
Follow-up test (6 weeks after start of therapy at the earliest)
|
Further follow-up testing (half-yearly)
|
Material LTT-FU or LTT-IS
20 ml heparin blood and 5 ml whole blood are needed for serum production.
(no cooling, no centrifugation needed)
The blood collection material is available free of charge as the “LTT blood collection set” from the laboratory, on request.
Requests
Please formulate your testing order on the referral form.
Test for immune function
without testing of BRMs
Request reads: LTT Immune function (LTT-FU)
Test for immune function
with simultaneous testing of BRMs
Request reads: LTT immunostimulators (LTT-IS)
Important:
For the LTT-IS, please note the products to be tested on the referral form (you can choose up to 6 products). We can provide you with a list of all BRMs available in our lab. Other products can be sent in together with the blood samples (Storage in the lab for follow-up testing as well as senderspecific profiles can be arranged over the phone).
Invoicing
Please obtain the costs from the pdf-document. The medical report will be sent to the sender’s practice within 10 days.
