Indication and interpretation of SARS-CoV-2 antibody diagnostics
The determination of antibodies against SARS-CoV-2 in serum does not replace the direct detection of the virus by PCR. This direct pathogen detection remains the gold standard for the detection of acute infection. However, very sensitive and sufficiently specific antibody tests are now available for laboratory diagnostics (in contrast to the very confusing market of rapid tests). There are two indications for the detection of antibodies against SARS-CoV-2:
1. Regarding the acute detection of infection in addition to direct PCR
As Fig. 1 shows, the viral load drops just a few days after the first symptoms appear. The sensitivity of direct detection of infection by PCR thus decreases significantly from day 7 onwards. This explains why the detection of IgM or IgA antibodies in the blood significantly increases the sensitivity of infection diagnosis in the early phase, i.e. from about one week after the onset of symptoms (Guo L, Clin Infect Dis. 2020; Zhao J, Clin Infect Dis. 2020).
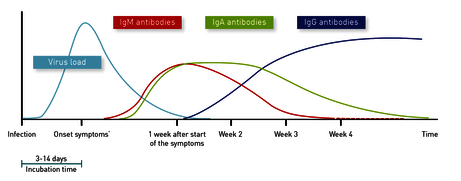
Fig. 1 Development of the antibody response in relation to the viral load and the time of symptom onset
IgM and IgA are early antibodies
The typical constellation for an acute or recent infection would be IgM/IgA positive, IgG (still) negative (see Fig. 2). The most common tests usually detect either IgA or IgM in addition to IgG. Our experience with parallel use of these tests shows that IgM is positive approx. 2 days earlier, but IgA remains positive for a longer time, so that both variants have advantages and disadvantages, but no different interpretations were found.
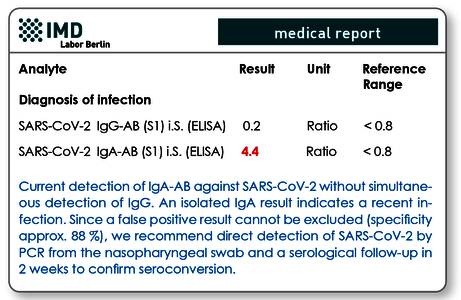
Fig. 2 Typical antibody findings in acute infection
2. Detection of antibodies to detect an infection that has occurred
The second indication for antibody diagnostics is to clarify whether a SARS-CoV-2 infection has already been experienced. For this purpose, IgG is of particular importance, which is only formed in the course of the advanced immune response after initial contact with the virus and, as with other viruses, persists for a very long time, possibly even permanently (see Fig. 1). Approx. 6 weeks after infection, 94 - 98 % of those affected show IgG antibodies. Parallel measurement of IgA can nevertheless be useful, as this method can also detect cases with delayed IgG formation (phenomenon of late IgG formation with mild symptoms! see Fig.1).
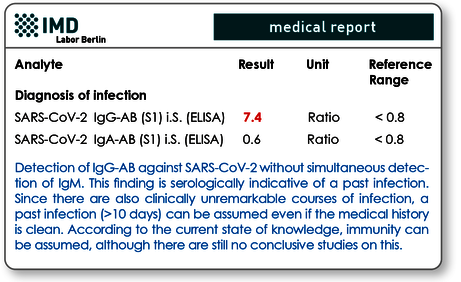
Fig. 3 Antibody findings with expired (previous) infection
The sensitivity of the antibody tests depends on the time of examination
The formation of IgM begins on the 3rd to 4th day after the onset of symptoms. In about 90 % of infected persons IgM is detectable in serum seven days after onset of symptoms. Various studies indicate that after two weeks, 100 % of patients have measurable IgM titers (Xiao AT, I Infect 2020). Other authors report that IgM was not detectable in 10 % at any time of infection (Padoan A, Clin Chem Lab Med 2020). How long the IgM persists in the blood seems to depend on the sensitivity of the test kit used. Some authors state that the peak of IgM formation is reached in the 2nd week (Sun B, Emerg Microbes Infect 2020), other study groups have observed that IgM does not decrease again until after 4 weeks (Xie J, J Med Virol. 2020).
IgA becomes measurable in serum 3-6 days after onset of symptoms (Guo L, Clin Infect Dis 2020). Compared to IgM, it occurs only slightly later, but persists longer and can therefore close the "gap" to IgG in some cases.
The IgA is positive earlier compared to the IgG (Jääskeläinen AJ Euro Surveill 2020). 7 days after the onset of symptoms about 50 % of patients show IgA levels, after 14 days the figure is 95 % (manufacturer's data Euroimmun). Compared to IgG, the first 10 days (early phase of the infection) show a higher sensitivity (Okba N, Emerg Infect Dis. 2020). Like IgM, IgA can persist for many weeks in individual cases (Padoan A Clin Chem Acta 2020).
IgG formation begins 7-10 days after the first symptoms appear. In the 1st week the sensitivity is only about 30 %. From the 3rd week onwards IgG reaches a sensitivity of about 94 % (manufacturer's data Euroimmun). In almost all published studies there were cases in which no IgG antibodies appeared even after several weeks despite PCR-assured infection. The frequency of this seronegativity is below 5 %. The dynamics of antibody formation is very individual in all three classes. The times given can therefore only represent average values.
The specificity of the antibody tests
If IgG were detected, how certain could we be that the person has actually had the infection?
This question and the terms of specificity and positive predictive value are currently the subject of heated debate in both professional circles and the media. Diagnostic specificity indicates the probability of finding an inconspicuous test result in a homogeneous group of healthy people. The information provided by the manufacturers for IgG is usually over 98 %, in some cases even close to 100 % accurate. This would mean that every IgG positive was in fact already infected, and therefore there are almost no false positive results. Unfortunately, however, this does not correspond to reality, because when the (analytical) specificity is stated, the influence of the division into sick and healthy persons in the examined group is often not taken into account (pre-test probability).
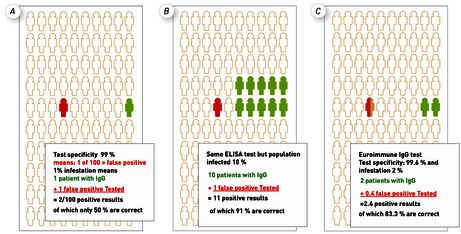
Fig. 4 A On an ELISA test kit with an analytical specificity of 99 %, one in 100 people tested is false positive (red). Assuming a 1 % population infection, IgG would be statistically right-positive (green) in a second of the 100 tested. This means that out of 2 patients tested IgG positive, one patient (50%) would be false positive. Fig. 4 B shows that with the same test, the positive predictive value increases to 91 % if a population with a 10 % screening is examined. Fig. 4 C reflects the estimated reality with the ELISA test kit from Euroimmun (specificity with 99.6 %) and the assumption of a 2 % screening in one region. The predictive value here would be 83.3 %, i.e. almost 2 out of 10 people tested would have a false-positive IgG.
As long as a disease is rare in the population (and this is currently still the case with SARS-CoV-2), the so-called positive predictive value reflects the reality better (see Fig. 4). For example, with an assumed infestation of 2 % and an analytical test specificity of 99.6 % (data for IgG EUROIMMUN) it can be calculated as 83.3 %. This means that statistically only 8.3 of 10 IgG-positive patients are actually positive. However, this is unavoidable and quite acceptable if it is taken into account in practice. In general, no serological or laboratory test can be 100 % specific and sensitive. It can only ever confirm or refute a suspicion (see also Box 1).
|
Box 1 Definitions of terms
Positive IgG should be confirmed
It is common practice in infection serology that positive screening tests must be confirmed by e.g. immunoblots or other more specific tests. This option is also available for the detection of SARS-CoV-2 IgG antibodies. In this test the "antibody profile" is determined, i.e. IgG antibodies against the S1 and S2 spike proteins and the nucleocapsid protein are analysed in parallel from a serum sample. Based on the number of positive results and the cut off values achieved, a positive IgG can be identified with much greater certainty as true positive or false positive.
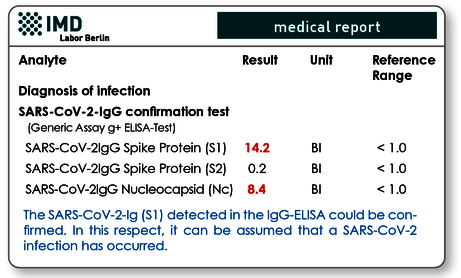
Fig. 5 Example of IgG confirmation test. The positive IgG (S1) result of the screening test was confirmed due to the significantly high IgGs against S1 protein and nucleocapsid.
Can one rely on IgM and IgA?
Especially IgM but also IgA show a higher avidity due to their polymeric structure (several binding arms, see Fig. 4). This means that they are "stickier", a property that helps to compensate for the still missing fit to the virus in the early phase of an infection. The disadvantage of this biologically useful property is that they are also "stickier" in the laboratory tests and therefore more often cause false positive results, especially compared to IgG.
According to the current data situation, false positive results can be expected in about 12-14 % of cases, regardless of manufacturer. Therefore, the diagnosis of Covid-19 should never be made on the basis of positive IgA or IgM detection alone, but always on the basis of direct PCR and (especially from the 2nd week after the onset of symptoms) clinically and radiologically supported (CT lung). A control examination at intervals of 14 days normally provides clarity, since in the case of an actual infection IgG (seroconversion) is then formed.
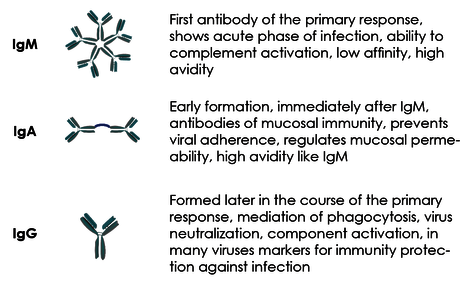
Fig. 6 Structure and function of the antibody classes
Antibody formation in patients with absent or mild symptoms
In most studies, patients with severe symptoms were examined. Cases that showed mild symptoms or were asymptomatic usually show delayed antibody formation as well as lower titers in all three antibody classes. In practice, this should be taken into account when examining a suspected acute infection, or when trying to detect a previous infection (To Kk, Lancet Infect Dis. 2020; Zhao et al; Clin Infect Dis. 2020 Mar 28).
Persistent IgA and IgM detection is possible
It is also known from other viruses and bacteria that in some patients both IgA and IgM antibodies can persist for many weeks. In a Chinese study of SARS-CoV-2 infected patients, IgM was positive in as many as 33 % of patients after 7 weeks. This could be due to unspecific reactivity of these two antibody classes (see below) or could be based on actual persistence. As soon as IgG is also positive, this IgM persistence is most likely no longer an indication of persistent infectivity. Up to now it has been assumed that the formation of IgG (i.e. seroconversion).
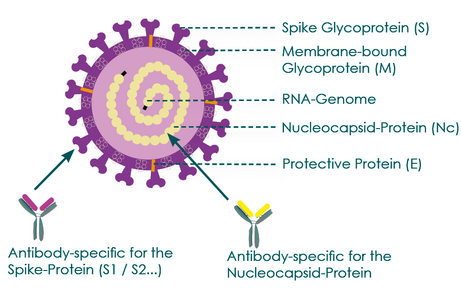
Antibodies can be directed against different virus components
Fig. 7 Representation of possible SARS-CoV-2 antibody binding sites
The detection of antibodies in patient serum is carried out with so-called enzyme immunoassays, usually ELISA tests. As SARS-CoV-2 specific binding proteins, different components of the virus are used, depending on the manufacturer, which can partly explain different results. In current SARS-CoV-2 antibody tests, either the spike protein (S1 domain) or the nucleocapsid protein (Nc protein) is used as a specific target antigen. Fig. 6 shows that the nucleocapsid protein is a protein that is located inside the virus and surrounds the virus' RNA genome. S1 proteins are proteins on the virus surface. They are the proteins with which the virus binds to the ACE2 receptor of human target cells in order to enter them.
For patients with a lack of antibody formation in a used test despite urgent clinical or anamnestic suspicion, a control with an ELISA containing the respective other protein as target antigen might be useful. For this reason, we have decided to use one test kit with S1 target antigen (EUROIMMUN) and one with Nc protein (Abbott) in our laboratory, so that we can clarify questionable results within the laboratory without complications.
Does antibody detection also mean immunity?
This is not certain because it cannot yet be proven by casecontrol studies due to the shortness of the virus' existence. Experiments on rhesus monkeys indicate at least a temporary immunity (infection protection) after primary infection (Bao, L. et al. Preprint at bioRxiv). Furthermore, research has shown that there is a correlation between the titer of neutralizing antibodies in the virus neutralization test and the serum antibody titer against both S1 proteins and nucleocapsid protein (Nc). It is important to note that a positive SARS-CoV-2 IgG result indicates that an infection has occurred, but this does not necessarily mean that infection protection (immunity) is associated with it. For this statement the necessary long- term studies are yet not available.
Immunity also possible without antibody formation
In our own patient collective, but also in various studies, it has been observed that in about 3 % of cases, patients with PCR- assured infections do not produce antibodies even after weeks. This can be caused by immune defects or atypically delayed antibody formation. If the infection has been confirmed, immunity may still exist, since the antibodies contribute to the immune defence of the virus (e.g. by neutralisation, i.e. inhibition of uptake into target cells), but the immune defence against the virus is primarily carried by the T-lymphocytes. This explains why in many cases the virus is completely eliminated before antibodies are detectable in the patient's blood. Corresponding cellular tests are currently used in research, but are not yet available for laboratory routine. It is also currently believed that the specific T-cell immunity (based on cross-reactivity) is responsible for children and some adults surviving the infection without symptoms of primary infection. What is certain is that this "immunity" is not based on antibodies.
Material required
The laboratory test for SARS-CoV-2 antibodies is carried out on 2 ml of serum. The determination of IgG and IgA antibodies is recommended for all indications. In case of acute infections the determination of IgM is also recommended.
Literature
- Guo Li et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin Infect Dis. 2020Zhao J, Clin Infect Dis. 2020 • Xiao AT, Gao C, Zhang S. Profile of specific antibodies to SARS-CoV-2: The first report. J Infect. 2020
- Padoan A et al. Analytical performances of a chemiluminescence immuno assay for SARS-CoV-2 IgM/IgG and antibody kinetics Clin Chem Lab Med.2020
- Xie J. et al. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J Med Virol. 2020
- Sun B, Feng Y, Mo X. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients.Emerg Microbes Infect. 2020
- Jääskeläinen AJ et al. Evaluation of commercial and automated SARSCoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples. Euro Surveill 2020
- Okba N et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Anti body Responses in Coronavirus Disease 2019 Patients. Emerg Infect Dis. 2020
- To Kk et al. Temporal Profiles of Viral Load in Posterior Oropharyngeal Saliva Samples and Serum Antibody Responses During Infection by SARS-CoV-2: An Observational Cohort Study, Lancet Infect Dis. 2020
