LTT for environmental pollutants and moulds
The lymphocyte transformation test (LTT) has proven to be superior in diagnosis of medication allergies compared to the epicutaneous test (1). This led to recommendation of the LTT for this question in the diagnostic guidelines of the German Society for Allergology and Clinical Immunology (DGAKI) (2).
On the other hand, the validity of LTT to detect sensitisations to xenobiotics and moulds is still considered questionable from the ‘official point of view’ although no sensible explanation is given for why the LTT should be less specific or sensitive for these allergens despite the identical test mechanism. The epicutaneous test is traditionally still recommended, although a laboratory test would be better suited for toxic and carcinogenic substances in particular than an epicutaneous test in which these substances are applied directly to the skin.
The LTT not only indicates the exposure.
It is not uncommon to hear that positive results from the LTT for environmental pollutants or moulds ‘only indicate exposure’ which is not always necessarily associated with clinical symptoms. A sensitisation detected in the LTT (but also in the skin test!) is not actually absolutely associated with clinical symptoms of inflammation. This does not cast doubt on the present sensitisation, however. It is correct that not every sensitisation at every point in time also produces allergic symptoms. Nevertheless, positive LTT results do not ‘only indicate exposure’. Otherwise, the rate of positive responses to metals such as nickel or the cadmium present in cigarette smoke, for example, would be far higher. The prevalence of positive responses in the LTT is far below the high number of people with relevant exposure.
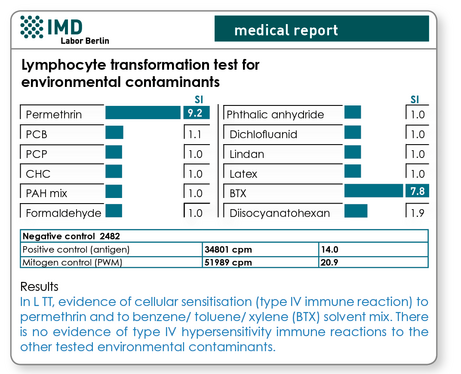
Fig. 1 Sample results for LTT for environment pollutants
The methodological studies on the LTT are often carried out using nickel because affected patients are easily available. To detect a type IV sensitisation to nickel, the LTT is undisputedly the tool of choice (3). At the Institute for Clinical Immunology of the University Hospital Essen, the correlation between the various test methods LTT, epicutaneous test and cytokine analyses and the clinical results was studied. There was an outstanding correlation of the results of the LTT, the epicutaneous test and the cytokine analyses. Compared to the clinical picture both the LTT and the epicutaneous test correlated (epicutaneous test, r=0.73, p<0.0001; LTT r=0.74, p<0.0001).
For intolerances to perfume (5), iodine-containing contrast agent (6) and methacrylates (7) and plasticisers it has been demonstrated that the LTT is suitable for the often problematic differentiation between allergic and irritative reactions.
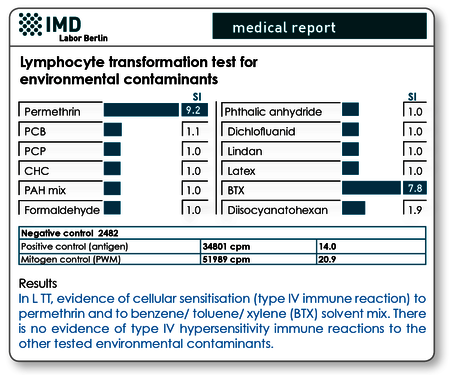
Fig. 2 Sample results for LTT for moulds
The quality of the test procedure is decisive
All recent studies show that the validity of the LTT results determined is more dependent on the quality of the test procedure than on the methodology itself. The LTT technologies used today in specialist immunology laboratories are very reliable and are characterised by high sensitivity and specificity. Continuous developments in cell culture techniques, the quality of the allergens used for cell stimulation and not least the use of genetically engineered interferon-α as an additive in the cell culture have contributed to this (8).
For preventive questions only the LTT should be used!
The epicutaneous test should not in principle be used for preventive testing of type IV sensitisations, because there is a risk of potential sensitisation as a result of applying the test substance to the skin (9). Agrup showed in one study in which epicutaneous tests were carried out twice for the standard allergens that with repeated testing there was a significant number of new sensitisations after 6 months. The prevalence of iatrogenic sensitisation is 5% for cobalt, 4.6% for p-phenylene diamine, 2.3% for chrome and 9.9% for p-aminoazobenzene, for example. Additional documented case descriptions are available for benzoyl peroxide, butyl hydroquinone, composite mix, 4-tert-butyl catechol, various plant extracts, budesonide, formaldehyde, nickel and acrylates.
Confirmation of a positive LTT result using an epicutaneous test is proscribed because the clinical symptoms may be amplified due to exposure to the test contact allergen. The limited sensitivity and lack of reproducibility of the epicutaneous test limits indications from such ‘follow-up testing’ in any event.
Experience gained over more than 10 years shows that a standardised procedure for the LTT is important for the following questions in particular:
- Negative epicutaneous test result with urgent clinical suspicion of a contact allergy
- Dubious positive results in the epicutaneous test (toxic reactions?)
- Preventive testing (e.g., before using dental restoration material) or queries from professional organisations
- Testing of potentially sensitising or carcinogenic substances
Literature
- Merk HF. Diagnosis of drug hypersensitivity: lymphocyte transformation test and cytokines. Toxicology 2005;209: 217-20
- Leitlinie zur In-vitro- Allergiediagnostik der Deutsche Gesellschaft für Allergologie und klinische Immunologie (DGAI) in Abstimmung mit der Deutschen Dermatologischen Gesellschaft (DDG) Journal der Deutschen Dermatologischen Gesellschaft (2006) 4; 72-85
- Hallab NJ. et al. Lymphocyte transformation testing for quantifying metal-implant-related hypersensitivity responses. Dermatitis. 2004;15:82-90.
- Lindemann M et al. ELISpot: a new tool for the detection of nickel sensitization. Clin. Exp. Allergy 2003;33:992-8
- Sieben S et al. Characterization of T cell responses to fragrances. Toxicol Appl Pharmacol. 2001;172:172-8
- Kanny G et al. T cell-mediated reactions to iodinated contrast media: valuation by skin and lymphocyte activation tests. J Allergy Clin Immunol. 2005;115:179-85.
- Kanerva L et al. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-8
- von Baehr et al. Improving the in vitro antigen specific T cell proliferation assay: the use of interferon-alpha to elicit antigen specific stimulation and decrease bystander proliferation. J. Immunol. Methods 2001; 251: 63-71.
- Agrup A., Sensitization induced by patch testing Br.J. Derm. 1986; 80;631-634
Numerous environmental pollutants and moulds can be tested as standard in the LTT. To simplify the laboratory request, the most important allergens are grouped in profiles which in some cases have overlapping contents.
| LTT MCS Environmental factors | Nickel, mercury, latex, PCP, PCBs, permethrin, formaldehyde, methyl meth-acrylate, Aspergillus fumigatus, Penicillium notatum, phthalic acid anhydride, dichlofluanid, PAH mix, 1,6-diisocyanatohexane |
| LTT Environment Pollutants | Formaldehyde, BTX, CHCs, lindane, PAH mix, PCBs, PCP, permethrin, latex, 1,6-diisocyanatohexane, phthalic acid anhydride, dichlofluanid |
| LTT Moulds | Aspergillus fumigatus, Penicillium chrysogenum, Trichophyton mentagrophytes, Cladosporum herbarum, Mucor mucedo, Alternaria alternata, Rhizopus nigricans, Botrytis cinerea, Stachybotrys atra and the yeast Candida albicans |
| LTT Flame Retardants | Tris(2-chloroethyl) phosphate (TCEP), tris(2-butoxylethyl) phosphate (TBEP), tris(2-ethylhexyl) phosphate (TEHP) |
| LTT Plasticisers | Phthalic acid anhydride, diethyl phthalates, dimethyl phthalates, dibutyl phthalates, dioctyl phthalates |
The German Professional Association for Environmental Medicine Specialists e.V. published a statement in May 2006, ‘The importance of epicutaneous tests and lymphocyte transformation test in the diagnosis of type IV sensitisations’. Journal of Laboratory Medicine 2006; 2: 101–106
If you are interested in the full text version, we would be happy to send it to you.
Please contact our serviceteam: +49 (0)30 770 01-220.
