Lyme Disease - Clinical symptoms and diagnostics
Epidemiology and aetiology
In Germany, about 10 % of nymphs and 10 - 40 % of adult ticks carry Borrelia bacteria. After being bitten by an infected tick, about 10 - 20 % of people develop an infection. There is a high risk of transmission if the tick has fed on the person’s blood for more than 48 hours. Tick bites are often not noticed, particularly if the person is bitten by nymphs, which cause most infections in humans.
Borrelia infections can proceed asymptomatically (pathogen elimination = spontaneous healing) or symptomatically (Lyme disease). In symptomatic infections about 60 - 80 % of people develop erythema migrans after being bitten by a tick.
In 20 - 40 % of people, Lyme disease manifests itself only in the disseminated stage. A Borrelia infection does not confer immunity. It is therefore possible to be reinfected. Prophylactic vaccination is not yet available.
Pathogens in Europe are:
- Borrelia burgdorferi sensu stricto – particularly with arthritis
- Borrelia garinii – particularly with involvement of the nervous system
- Borrelia afzelii – particularly with late skin manifestations
Transmitters of Borrelia bacteria in Europe are ticks of the Ixodes genus (Ixodes ricinus).
Stages and clinical findings
1. Early localised Lyme disease – erythema migrans
Erythema migrans starts 2 to 40 days following the tick bite with a small red papula or a simple reddening of the skin developing at the site of the bite which then spreads centrifugally over days or weeks, often accompanied by local itching and burning. The edge is usually emphasised and the morphology is highly variable. There are also homogeneous, non-migrating forms. Predilection sites are skin folds (groin, back of knee, armpit) and in children, the head and nape. The erythema migrans is often accompanied by general flu-like symptoms (“Borrelia flu”).
Diagnostics with suspected primary infection
Critical here is the history of a tick bite and existing erythema migrans. Pathogen-specific antibodies can be expected about 3 to 8 weeks after the infection. The antibiotic therapy must therefore be initiated without serological diagnostics if the patient is displaying typical clinical symptoms.
With atypical clinical results, it is recommended to examine the serum for antibodies against Borrelia burgdorferi, noting that there is a diagnostic window of up to 8 weeks after the bite. The new Borrelia recomBead test is an alternative to the conventional procedure (ELISA + Borrelia Western blot). If the tick that caused the bite is available (even dead), it can be examined to determine if it carries Borrelia bacteria using PCR. With a negative PCR result, Borrelia transmission to the patient is less likely.
Differential diagnoses of erythema migrans:
- Erysipelas
- Local reactions to tick bite or bite of other insects (usually occur immediately after the bite)
- Tinea corporis – adverse drug reaction, contact dermatitis granuloma annulare
- Urticaria, erythema exsudativum multiforme
Therapy recommendation
We refer here to the guidelines from the German Lyme Disease Association (Deutsche Borreliosegesellschaft, DBG), available at www.borreliose-gesellschaft.de, or the guidelines of other professional associations.
2. Early disseminated Lyme disease
If the Borrelia bacteria are not spontaneously eliminated or persist due to inadequate or incomplete antibiotic treatment of the erythema migrans, the pathogen disseminates, originating from the local infection at the bite site. Erythema migrans was not noticed by about 20 - 40 % of patients with early disseminated Lyme disease. The symptoms start usually about 1 to 4 months after the tick bite.
General symptoms:Flu-like symptoms, fatigue, myalgia, arthralgia, headaches, slight fever, swelling of the lymph glands, neck stiffness, back pain, loss of appetite
Skin: Multiple erythema migrans lesions; Borrelial lymphocytoma (cutaneous lymphoid hyperplasia): occurs 1 to 2 months after infection; localised blue-red nodes (often on the ear lobes, nipples, scrotum or nose) with soft, elastic consistency; often accompanied by regional lymph node swelling
CNS: Meningitis, cranial nerve dysfunction (often facial nerve paralysis), meningoradiculoneuritis (Bannwarth’s syndrome), transverse myelitis; Heart: Perimyocarditis, usually noticed as first to third degree AV block; very rarely chronic inflammatory (dilated) cardiomyopathy
Joints: Acute migratory arthralgia or transient joint swelling (episodic arthritis), often monoarticular or asymmetric oligoarticular arthritis
Eyes: Iritis, uveitis, chorioiditis, episcleritis/scleritis, orbital myositis, papillitis, retrobulbar neuritis
Diagnostics with suspected disseminated Lyme disease
Critical for the diagnosis are the medical history (tick bite? erythema migrans? risk behaviour?) and the current clinical symptoms.
Laboratory diagnostics:
Serological examinations can facilitate the diagnosis but never confirm or exclude it. Complicating matters is the fact that the ELISA and even the blot tests used in different laboratories have not yet been standardised. Usually the IgG/IgM screening test is carried out. A positive screening test must be confirmed in the immunoblot due to a high rate of false positive results. Since 2011, Borreliaspecific antibodies can also be serologically verified using the Borrelia recomBead test (multiplex procedure).
The lymphocyte transformation test (LTT Borrelia) can be helpful particularly for questionable or positive serology as well as for uncertain clinical symptoms and medical history (residual titre or active infection?).
Verifying the presence of the pathogen by propagating it from a skin biopsy, CSF and blood is only of minor importance in daily clinical practice (low sensitivity, high costs). Borrelia bacteria verification using PCR can be helpful for joint punctures and biopsies (especially from the affected areas of the skin). Blood and urine are not suitable because both false positive and false negative results occur.
Differential diagnoses:
- Erythema exsudativum multiforme
- Meningitis of other origin (e.g., viral, other bacteria)
- Facial nerve paralysis of other origin (e.g., VZV infection, idiopathic)
- Radiculoneuritis: prolapsed disc, Herpes zoster (preeruptive), Guillain-Barré syndrome
- Carditis: rheumatic fever, viral myocarditis
- Arthritis: other arthritis causes, particularly reactive or purulent arthritis.
Therapy recommendations:
See guidelines from the DBG. www.borreliose-gesellschaft.de or those of other professional associations.
The therapy is monitored clinically for the main part. With suspected ‘therapy failure’ or re-infection, LTT with Borrelia antigens (if there is a previous positive examination), or repeat examination of a CSF/serum pair can be helpful. Antibody confirmation using blood is unsuitable to assess the success of the therapy because the relevant antibodies persist in the serum for long periods.
3. Chronic Lyme disease (late manifestations)
The symptoms start about 4–6 months to several years after the initial infection. Classification of the diverse symptoms to a past infection with Borrelia bacteria is often difficult.
„Lyme-arthritis“ manifests on average more than 6 months after the initial infection (erythema migrans? tick bite?). It has a chronic recurrent course and usually affects large joints (often the knee joints) with swelling and pain.
Manifestations of chronic Lyme disease / neurological Lyme disease:
- Encephalomyelitis (neurological dysfunction, insidious deterioration of condition)
- Encephalopathy (impaired memory and concentration, cephalgia, tinnitus)
- Sleep disorders, depression, irritability, fatigue
- Normal pressure hydrocephalus
- Cerebral vasculitis, cerebral infarction
- Chronic radiculoneuropathy
„Acrodermatitis chronica atrophicans“ (ACA) is a chronic inflammatory, in part oedematous process, usually on acral skin regions exposed to the sun (often the hands). The chronic inflammatory stage is followed by the chronic atrophic stage (parchment-type skin with typical histological findings).
Concomitant symptoms include hyperaesthesia, muscle weakness, muscle cramps, isolated or multiple fibrous nodes and regional or generalised lymph node swellings.
Chronic ophthalmic Lyme disease manifests under some circumstances with corneal stromal opacities, marginal keratitis, episcleritis, ocular myositis and optic atrophy.
Diagnostics for chronic Lyme disease or late manifestations
The diagnosis is verified using serological analysis or for clinically suspected chronic neurological Lyme disease, by analysing a CSF/serum pair, possibly using the LTT. For acrodermatitis chronica atrophicans, a histological examination can also be done.
Differential diagnoses:
- Lyme arthritis: rheumatoid arthritis, fibromyalgia
- Chronic neurological Lyme disease: other inflammatory neurological diseases (e.g., viral, multiple sclerosis)
- ACA: skin changes due to venous insufficiency, scleroderma, lichen sclerosus
Therapy recommendations:
See the guidelines of the DBG www.borreliose-gesellschaft. de. Other medical societies have so far scarcely commented on the treatment of chronic borreliosis.
Therapy monitoring has been primarily clinical (note: improvement is usually gradual) or via LTT-Borrelia if there was a previous finding. Renewed examination of a liquor-/ serum pair is also advisable. Verification of antibodies in the blood is not suitable for monitoring the course.
As a sign that a patient has developed chronic Lyme disease a reduced number of CD57-positive natural killer cells is shown in the blood. Whereas during acute Lyme disease and other illnesses normal CD57-NK values were measured, according to Stricker et al., patients with acute Borrelia infection often have values below 60 CD57+ NK cells/µl blood.
4. Post Lyme disease syndrome (PLDS) / chronic Lyme disease
This is a syndrome that persists after antibiotic treatment of Lyme disease, in some cases even after repeated treatment. Pathogenetic causes being considered include a protracted (auto)immunological activation and Borrelia-induced vasculitis.
PLDS or chronic Lyme disease? Antibiotic treatment – yes or no? These questions are hotly debated by specialists. The available laboratory diagnostic options have not yet been able to resolve this contentious issue.
The most common symptoms include fatigue, exhaustion, cognitive deficits and sleep disorders, neuropathies, pain syndromes.
The following are helpful for diagnostics: medical history (previous Lyme disease?) and serological results (past infection?).
For differential diagnostics (particularly to differentiate from fibromyalgia or non-specific joint and systemic diseases), HLA-DR subtyping (see page 4) and LTT Borrelia are also recommended. The LTT is negative if there are no active Borrelia infection present but it does not exclude PLDS. A positive LTT supports the suspicion of a persistent Borrelia infection.
The question of whether long-term antibiotic therapy is promising is disputed controversial amongst specialists.
Special versions of neurological Lyme disease
Neurological Lyme disease is a result of disseminated (systemic) infection with Borrelia bacteria with the involvement of the nervous system.
Clinical symptoms
Meningopolyneuritis, polyradiculitis (Garin-Bujadoux-Bannwarth syndrome)
Weeks to months after infection (tick bite!)
- Burning radicular pain (mononeuritis)
- Asymmetrical sensory disorders
- Paralysis manifestations often with the involvement of cranial nerves
- Localisation often near the tick bite (medical history!)
- DD: also nerve root compression (disc prolapse)
Possible consequences of unknown Borrelia-related radiculitis include permanent pain symptoms, paraesthesia, temperature perception disorders and pain perception disorders.
Cranial nerve palsy
Weeks to months after infection (tick bite!)
Cranial nerves affected:
- Facial nerve (common, occasionally observed in children as facial diplegia)
- Abducens nerve (rarely)
- Oculomotor nerve, optic nerve
Differential diagnostics must be considered: bleeding, vascular occlusion, tumour, multiple sclerosis, neurosyphilis
Encephalitis, myelitis
are late sequelae of a Borrelia bacteria infection. The symptoms here are highly diverse:
- Cranial nerve palsy, gait disorders, paralysis
- Voiding disorders
- Epilepsy, character change, dementia
- Sleep disorders, impaired concentration
- Chronic fatigue, hallucinations, rarely psychosis
Differential diagnostics must be considered: multiple sclerosis, neurosyphilis, viral infection (e.g., tick-borne encephalitis)
Laboratory diagnostics for neurological Lyme disease
Primarily, a previous infection is verified (EIA for IgG and IgM and immunoblot with serum). With unclear serology and suspicious clinical symptoms a lymphocyte transformation test should be sought. Information on the sensitivity of the LTT in neuroborreliosis are not yet available.
The following are examined in the CSF:
- Cell count (analysis max. 1–2 hours after puncture)
- Total protein
- Albumin ratio (CSF/serum)
- Q-IgG, Q-IgM, Q-IgA (CSF/Serum)
- Oligoclonal bands (CSF/serum)
- Antibodies against Borrelia burgdorferi (antibody specificity index, ASI) (CSF/serum)
Typical CSF results for neurological Lyme disease
- Slightly elevated cell count and elevated total protein
- Albumin ratio: slight to moderate blood-CSF barrier function disorder
- Q-IgG, Q-IgM, Q-IgA: intrathecal synthesis (three-class reaction) with IgM antibody dominance
- Oligoclonal bands: only partly detectable
- Borrelia burgdorferi ASI: evidence of intrathecal antibody synthesis against B. burgdorferi
The laboratory results are interpreted taking the clinical details and medical history provided by the treating doctor into consideration. However, it must also be considered that about 50 % of tick bites are not noticed and only about 60 - 80 % of those infected develop erythema migrans.
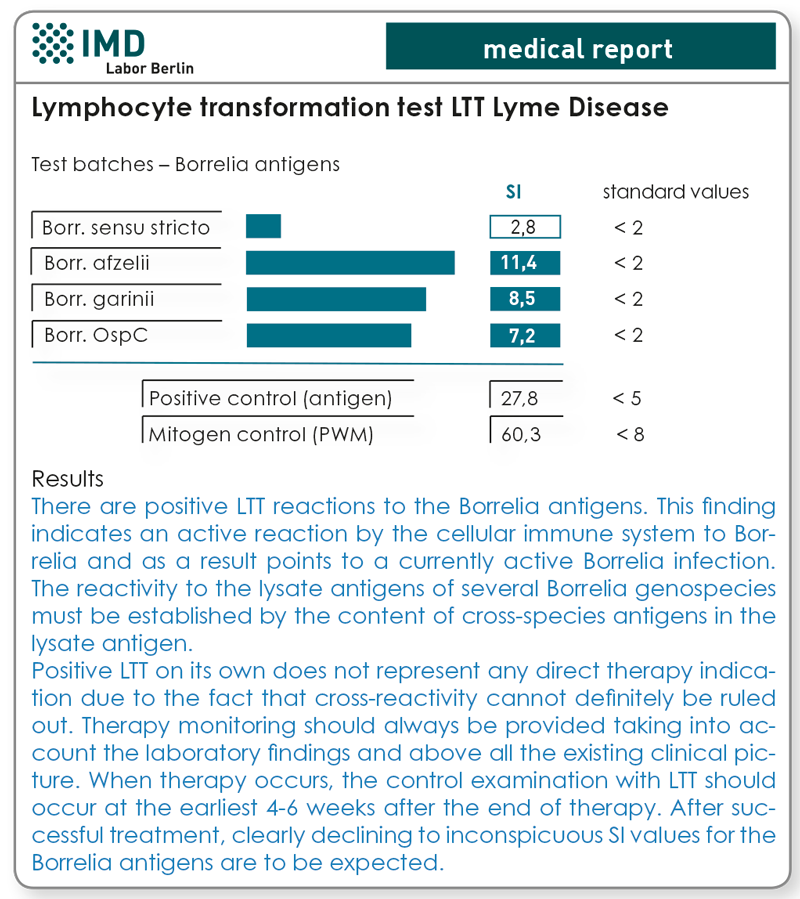
The lymphocyte transformation test (LTT)
Diagnosis of borreliosis should occur primarily according to clinical criteria. Please note that a not clearly definable number of clinically symptomatic cases at all stages cannot be clearly evaluated serologically and for this reason clinical diagnosis is also of the greatest importance.
Positive demonstration of Borrelia-specific antibodies merely shows that Borrelia infection existed previously. Whether the infection is still active at the time of the examination or the pathogens have been eliminated by the immune system or therapy cannot be determined with serological methods. Consequently, producing a diagnosis of borreliosis in stage II/III can be difficult.
The lymphocyte transformation test with borreliosis antigens (LTT-borreliosis) has been available since 2007 as an additional diagnostic option. The cellular immune response of lymphocytes to Borrelia proteins was demonstrated in this connection. The test is positive when Borrelia-specific T-lymphocytes exist in the patient’s blood. These indicate that at the time the blood was taken, the immune system was involved in an immunological reaction to the pathogen. If this is followed by an effective antibiotic treatment, there is a significant decline in the stimulation ratios (SI values) in most patients.
The examinations we have conducted at the Institute for Medical Diagnostics as part of the validation of LTT have shown a sensitivity of the method before antibiotic treatment of 89.4 %. In the case of seronegative patients/ test persons, the specificity was 98.7 % and in the case of seropositive patients 91.6 % (from Baehr et al. Open Neurol J. 2012;6:104-12). But this also means that LTT can also have no 100 %-specificity in the case of lege artis conducted methodology, which must be considered when evaluating the totality of laboratory findings.
A negative finding in the LTT borreliosis test does not definitely rule out an active infection. Therefore, the evaluation of the clinical picture should always come first in the diagnosis of borreliosis and the indication for treatment based on it.
Indications for the LTT Borrelia
- Clinically suspected borreliosis with inconclusive serological findings
- Therapy monitoring after antibiotic treatment
What can HLA determination achieve in the diagnostics and assessment of the course of Lyme disease?
Joint symptoms persist over months and years despite adequate antibiotic therapy in about 10 % of patients with Lyme disease. With such persistence of symptoms there is always the question of the cause. Was the therapy inadequate or was the diagnosis incorrect? Inconclusive laboratory diagnostics compound this issue.
The HLA type can indicate a predisposition for a chronic course of Lyme disease.
The cellular and humoral immune response to the outer surface protein (OspA) of the Borrelia bacteria has a critical influence on sensitivity to the therapy. People with HLA-DR2 or DR4 have a genetic predisposition to developing treatment-resistant Lyme disease (relative risk up to 22 times higher).
STEERE et al. were able to show significant associations between particular HLA-DR subtypes (DR*0101, *1501, *0401 and *0402) and the cellular and humoral immune response to certain Borrelia outer surface antigens (OspA). Apparently, OspA triggers a cross-reaction with the body’s own structures if it is presented on the HLA molecules indicated as part of the immune response. This molecular mimicry drives the inflammatory process by means of autoimmunological processes, even if the pathogen itself has already been eliminated.
Other HLA alleles could be the reason behind the lack of antibody formation.
Recent investigations have focused on the question of HLADR association with seronegativity for verifiable Borrelia infection (Borrelia PCR and positive culture). In some cases, patients do not develop specific antibodies against Borrelia burgdorferi after a previous Borrelia infection. Almost 40 % of seronegative Lyme disease patients are positive for HLADR1.
Summary of previous study results
HLA association with treatment-resistant borreliosis disease:
DR1 (HLA-DRB1*01:01)
DR2 (HLA-DRB1*15:01)
DR4 (HLA-DRB1*04:01, 04:02, 04:03, 04:04;04:05; 04:07)
HLA association in infected patients with reduced formation of Borrelia-specific antibodies:
DR1-Allele (HLA-DRB1*01:02, *01:01,*01:04, *01:05, 01:03)
Material
IgG/IgM-ELISA
5 ml whole blood for serum extraction
Borrelia-recom Bead Blot (IgG + IgM)
5 ml whole blood for serum extraction
LTT Borrelia
20 ml heparin blood + 10 ml whole blood
CD57 NK cells
2 ml EDTA blood
HLA-DR-subtyping for Lyme disease
2 ml EDTA blood
Costs
Please obtain the costs for the tests from the pdf-document.
Literature
- von Baehr V et al., The lymphocyte transformation test for borrelia detects active lyme borreliosis and verifies effective antibiotic treatment. Open Neurol J. 2012;6:104-12
- Bauer, Y et al.(2001) Prominent T cell response to a selectively in vivo expressed Borrelia burgdorferi outer surface protein in patients with Lyme disease. Eur.J. Immunol. 31; 767-776
- Berghoff W (2009) Klinische Symptomatik der Lyme Borreliose und der Neuroborreliose [Clinical symptoms of Lyme disease and neurological Lyme disease]. umwelt med ges. 22:104–111
- Deutsche Borreliose-Gesellschaft (German Lyme Disease Association): Guidelines on the diagnostics and treatment of Lyme disease. www.borreliose-gesellschaft.de/Texte/Leitlinien.pdf
- Dressler, F. et al. (1992) The T-cell proliferative assay in the diagnosis of Lyme disease. Ann. Intern. Med. 116:603
- Krause A et al.(1991) T cell proliferation induced by Borrelia burgdorferi in patients with Lyme borreliosis. Autologous serum required for optimum stimulation. Arthritis Rheum. 34: 393-402
- RA Kalish et al. (1993): Association of Treatment-Resistant Chronic Lyme Arthritis with HLA-DR4 and Antibody Reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 61:2774-79
- AC Steere at al. (1990): Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 Alleles. N Engl J Med. 323:219-223
- R.B. Stricker, E.E.Winger (2001): Decreased CD57 lymphocyte subset in patients with chronic Lyme disease.
- Immunology Letters 76; 43-48 P Wang, E Hilton (2001): Contribution of HLA Alleles in the Regulation of Antibody Production in Lyme Disease. Frontiers in Bioscience 6: 10-16.
