Immunological laboratory testing in patients with tumours
The success of an “immunostimulatory” therapy for patients with tumour diseases essentially depends on the following points:
1. Is immunostimulation indicated?
- Immunostimulation must be indicated, i.e., it must be proven that those functional immune parameters are actually reduced. The stimulation of a functionally intact immune system is only helpful in exceptional cases. The current immunocompetence can only be evaluated through functional tests (LTT immune function, NK cell cytotoxicity test) and only with that it can successfully be controlled afterwards.
- It must be excluded that a pre-existing immune activation is not the cause of a reduced lymphocyte or NK cell function. In the cellular immune profile, therefore, the HLADR+ T cells should not be substantially elevated and the CD4/CD8 ratio should not be lower than 0.8.
- It must be proven that the patient has sufficient immune cells for stimulation (espcially CD4+ helper cells). 250 CD4+ cells/µl are indicated as the limit. Otherwise, an immune-restorative therapy is preferable to immunostimulatory measures (trace elements, vitamins, thymus).
2. Is the immune system moving in the right direction during therapy?
During immunostimulatory therapy (e.g. with preparations containing mistletoe, organs or microorganisms), the following laboratory parameters can be consulted to evaluate progress and prognosis. Following questions can be addressed:
- Does the proportion of immunosuppressive regulatory T cells (Treg) remain low?
The regulatory T cells (Treg) are an important sub-group of the CD4 cells (normal approx. 4-10 %). They play a central role in the maintenance of immunotolerance, which is counterproductive in tumour illnesses. Regulatory T cells inhibit the effector functions of cytotoxic T cells, NK cells and other immune cells against tumour antigens and thus support tumour growth. A direct correlation between the number of Treg cells and the stage of the tumour could be detected. Advanced tumour stages showed an increased infiltration of the tissue with Tregs. In addition, an inverse correlation between the number of Tregs in the tumour tissue and survival rate could be pointed out.
Treg cells are well suited as progress markers in immunomodulating therapies. It is prognostically favourable if they do not rise. Regulatory T cells are phenotypically identified by flow cytometry based on the cell surface marker constellation CD4+ CD25++CD127low.
In addition, the surface molecule CD39 is identified on Treg cells. CD39 is a peripheral membrane protein (ectoenzyme), which causes the transformation of ATP and ADP into AMP. AMP is converted extracellularly into adenosine, which acts as an immunosuppressant. The detection of the CD39+ Treg fraction can thus give a clue about the current immunosuppressive capacity of the Treg cells.
- Do the CD8 cells with cytotoxic effector capacity increase?
The term CD8 suppressor cells which is used incorrectly even today for the entire CD8 population is out-of-date. The “CD28 Status” differentiates the CD8 lymphocytes in cells with cytotoxic properties and those that actually perform a suppressive (immunosuppressive) function. As a matter of course the goal of immunostimulatory therapy is to increase the proportion of cytotoxic i.e. CD28-positive CD8 cells.
- Do the activated natural killer cells increase?
It is prognostically favourable, that during immunostimulatory therapy activation markers such as CD25 (receptor for interleukin-2) increase on natural killer cells. However, this test does not replace analysis of the NK cell function.
- What is the patient’s residual thymic function like?
The surface marker CD31 defines a subpopulation of the naive CD4+ helper cells, which only recently left the thymus. These are referred to as “recent thymic emigrants – RTE cells”. The proportion of the CD31+ naive CD4 cells in the blood is thus a measure of the residual thymic function. A decreased thymic function is exhibited in a reduced “ability to replenish” virgin T lymphocytes if there is an intensified “consumption” due to infections or after chemotherapy or radiotherapy. Therefore, the test is useful in the event of therapies that are immunologically damaging to assess the regeneration capacity and possible planning of early intervention (immune restoration marker).
The parameters mentioned are included in the quantitative immune profile “Immunocompetence Tumour” in addition to the recognised standard analyses such as CD4, CD8, NK and B cells and activated T cells.
Which diagnostic procedure is recommended?
Before immunostimulatory therapy
- LTT immune function + NK cell cytotoxicity test (as an indication and a starting point)
- Quantitative immune profile “Immunocompetence Tumour “
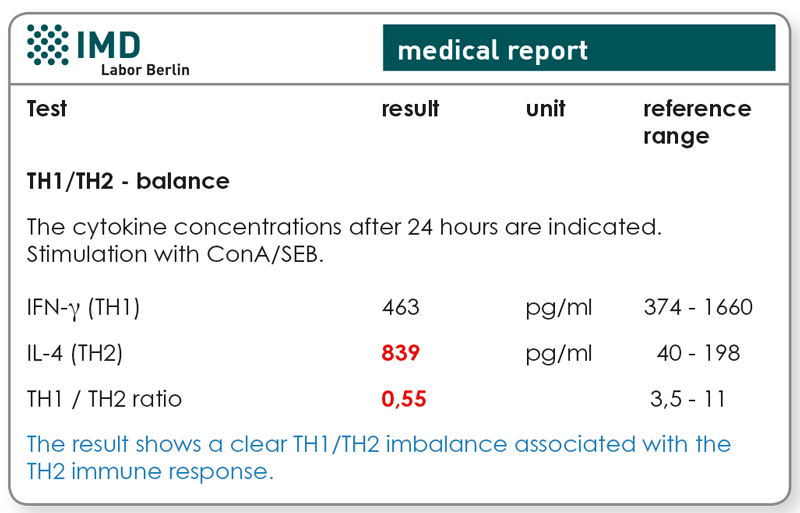
- TH1/TH2 profile (as initial value)
The repetition of the analyses 6-8 weeks after beginning immunostimulation answers the following questions:
- Did the function of the T lymphocytes and NK cells improve?
- Is there a shifts in the cell markers which point toward a sustained improvement in the immunological situation (esp. Tregcells and a portion of CD39+ Treg cells, CD28+ cytotoxic T cells)?
- Could a TH1 polarisation be achieved (IFN-γ increase and/or IL-4 decrease)?
Subsequently, while check-ups 4-8 months apart are recommended regardless of the clinical situation, one may be limited to the pathological findings in the initial diagnosis.
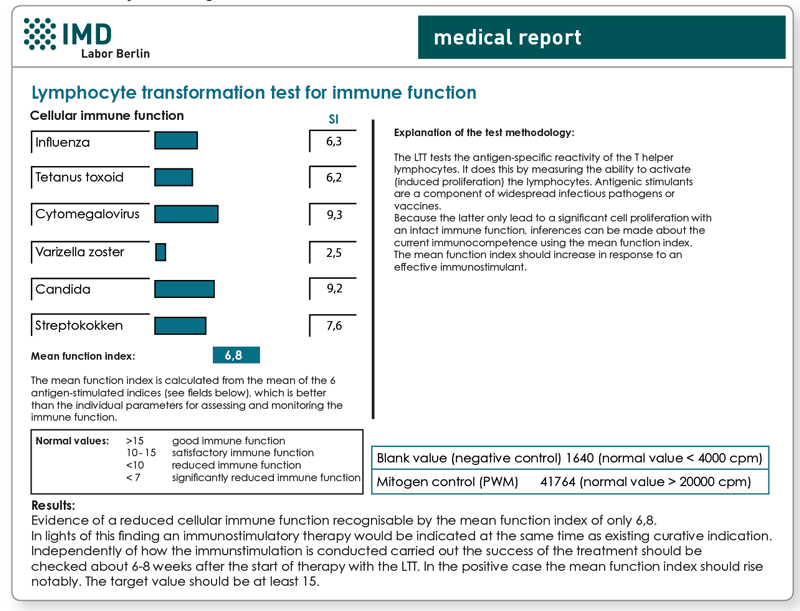
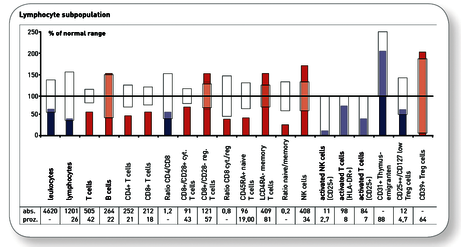
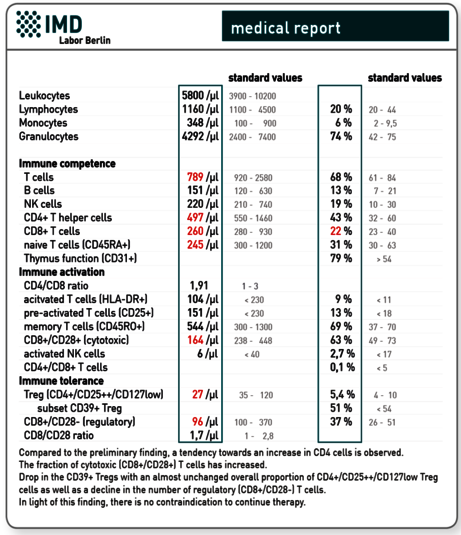
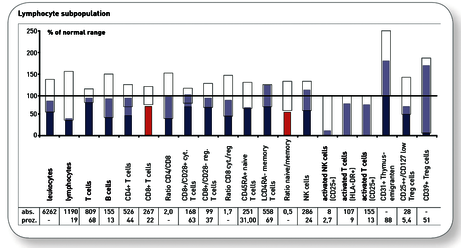
Explanatory representation of a very good progress finding after alternating therapy with mistletoe and a thymic preparation for a 54-year-old female patient with breast cancer.
1. LTT Immune Function
Preliminary findings
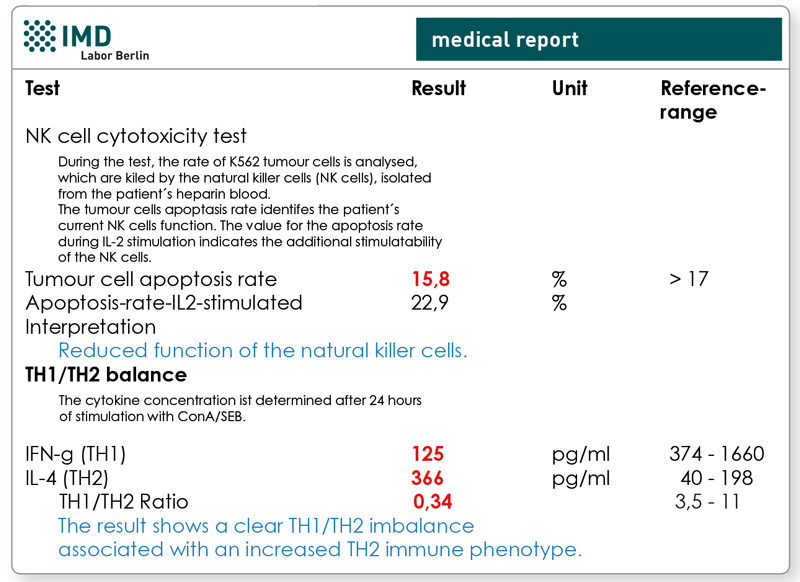
2. NK cell cytotoxicity test and TH1/TH2-Profile
Preliminary findings
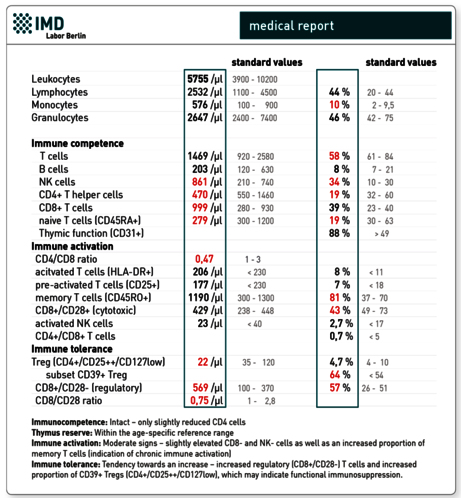
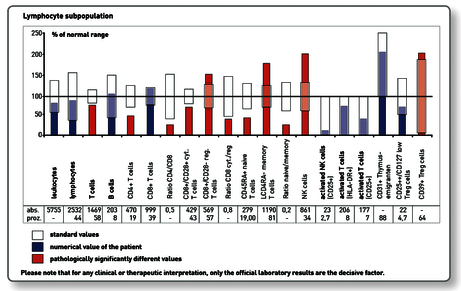
3. Immune profile “Immunocompetence Tumour”
Preliminary findings
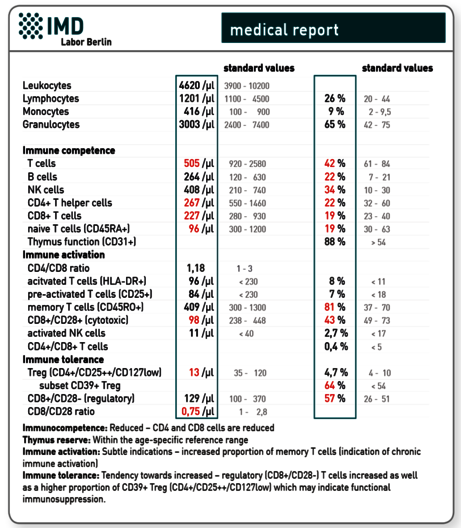
Medical finding at 6-week follow-up
Material
LTT Immune Function:
20 ml heparin blood + 5 ml whole blood (serum)
NK cell cytotoxicity test:
10 ml heparin blood
Quantitative immune profile “Immunocompetence Tumour “
2 ml EDTA blood
TH1/TH2-Profile:
5 ml heparin blood
Would you like to see a presentation on the matter?
In our video archive, you can find a free presentation on this topic. Access is free und possible without prior registration.
Literature
- AM Gallimore et al. Positive and negative influences of regulatory cells on tumour immunity. Oncogene 2008; 27:5586-93
- Kohler S, Thiel A. Life after the thymus - CD31+ and CD31- human naive CD4+ T-cell subsets. Blood. 2008; 26
- Vrisekoop N, van Gent R, de Boer AB, Otto SA, Borleffs JC, Steingrover R, Prins JM, Kuijpers TW, Wolfs TF, Geelen SP, Vulto I, Lansdorp P, Tesselaar K, Borghans JA, Miedema F.: Restoration of the CD4 T cell compartment after long-term highly active antiretroviral therapy without phenotypical signs of accelerated immunological aging. J Immunol. 2008; 15;181:1573-81
- Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R, Phair J, Jamieson BD. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008 1;180:1499-507.
- Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Müller CE, Murakami T, Robson SC.CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice.