LTT in allergology
Another indication for LTT is allergy testing
As the result of disturbed immune tolerance, allergies are on the rise in all advanced industrialized countries, in some parts with dramatic speed. Among these are the cell-mediated allergies (type IV). The detection of type IV sensitisations is another indication for the LTT.
The lymphocyte transformation test (LTT) is used in laboratory testing for:
- Drug Allergies
- Allergies to Dental Restorative and Implant Materials
- Individual sensitisation to environmental pollutants
- Type IV sensitisation to moulds
- non-IgE-mediated food intolerances type IV
Until about 1998, the lymphocyte transformation test (LTT) was used exclusively to assess immune function. Before this, the LTT was of little use in allergy testing, as the methods and technologies available then were not capable of detecting very low frequencies of allergen-specific T cells with the required certainty.
Only with the development of cell culture techniques and media, of radioactive analytical techniques and, not least, the use of methodological variations, such as adding recombinant interferon-α to the cell culture, the LTT evolved into a reproducible and highly sensitive method suitable for allergy testing.
The changes to the method, especially the addition of IFN-α to the test preparation, were already published in Journal of Immunological Methods in 1995 and are discussed in detail in this brochure’s general section about LTT methods.
Procedure
When the LTT for allergens is performed, mononuclear cells are isolated from the patient’s heparinised venous blood under sterile conditions using density gradient centrifugation. Following several washing steps, these cells are cultured together with the test allergens (e.g. medications, metals, environmental allergens, among others) under optimised cell culture conditions over a period of 5 days.
In patients with allergen-specific sensitisation, i.e. where lymphocytes specifically recognising the allergen circulate in the blood, these lymphocytes are activated to start clonal proliferation.
After day 5, 3H-thymidine is added to the cell culture for further 12 hours. This is integrated into the newly formed DNA of activated (dividing) lymphocytes.
Based on the content of 3H-thymidine-marked DNA, the cell division rate in the respective culture and thus the cell activation in this culture can be quantified.

To show the result, the allergen-induced lymphocyte proliferation is calculated in comparison with the spontaneous proliferation (blank) and the result is reported as stimulation index. The stimulation index is the ratio of the proliferation rates in the allergen-stimulated test run and the non-stimulated blank.
If the LTT is used to detect allergic sensitisations, SI values below 2 are regarded as negative (no sensitisation).
Results between 2 and 3 are regarded as borderline positive and should be followed up, where necessary.
SI values above 3 indicate a positive result. An SI >3 proves the presence of antigen-specific T cells in the patient’s blood (Evidence of an existing immunological sensitisation of the delayed type as the basis of a type IV allergy).
LTT versus epicutaneous test?
To detect type IV sensitisation, 2 independent test methods are available: the LTT and the epicutaneous test. For some questions, these complement each other well (e.g. in occupational dermatology).
The key difference between the two methods is that the epicutaneous test is only validated to detect contact allergies, i.e. for allergies where the sensitisation occurred by skin contact and which manifest on the skin.
Whenever allergens are absorbed via mucous membranes, this is referred to as systemic sensitisation. Here, the LTT offers significant advantages with regard to sensitivity. Because of this, the LTT was included as a test for the detection of sensitisation to medications in the allergologic guidelines and classed as "unreservedly recommendable” by the Guidelines Committee of the Robert Koch Institute.
Even though the dermatological professional associations have until today refused to acknowledge it, it is a fact that food, mould and restorative materials for dental work are absorbed through the mucous membranes and not through the skin.
Since the sensitivity of the epicutaneous test, depending on the test allergen, has only a sensitivity of less than 70%, a negative epicutaneous test result does no rule out a sensitisation.
The committee for “Methods and Quality Assurances“ at the Robert Koch Institute also reached this conclusion in its opinion on the LTT (Bundesgesundheitsblatt 2008; 51: 1070-1076) where it recommends, based on “socioeconomic reasons“, for metals and acrylates to “....first perform an epicutaneous test and then, in case of a negative result and continued clinical suspicion, an LTT ...“. This means that the LTT is regarded as the more expensive but ultimately more sensitive method.
Today, the LTT’s sensitivity is reported as approx. 90%, subject to the test allergen.
The specificity depends on the validation of each individual allergen and the quality of the performance of the LTT. At a DIN 15189-accredited laboratory, these sources of error can largely be excluded by internal standardisation.
The advantages and disadvantages of the two test methods were highlighted in the opinion of the German Professional Association of Physicians specialised in Environmental Medicine (see page 32-36). This opinion published in 2006 in the Journal of Laboratory Methods 30: 101-6 is still current.
Therefore, in case of preventative testing preference should always be given to the LTT, because even if the epicutaneous test triggers a sensitisation of the patient, this cannot be recognised in the corresponding epicutaneous test. It would take a second epicutaneous test performed 10-14 days later to detect this sensitisation. However, this “testing” is rather performed by the dentist or orthopaedic surgeon when they introduce the replacement material into the body.
Carcinogenic and toxic substances should never be administered to a patient’s skin. Therefore, they cannot be used with the epicutaneous test. Here, the LTT as an in-vitro method should be preferred.
The committee for “Methods and Quality Assurances“ at the Robert Koch Institute summarises the advantages of the LTT as follows:
|
At the IMD-Berlin, the LTT was accredited according to DIN 15189 as early as schon 2004 and has since then been audited by the Accredited bodies (DAkkS) every 2 years. The requirements for laboratory accreditation according to DIN 15189 significantly exceed those for ISO certification, because here expert reviewers also evaluate the indications, the establishment, the validation, the conduction of ring trials, and the result interpretation of an analytical method.
LTT - Medications
The lymphocyte transformation test (LTT) in laboratory testing for drug allergies
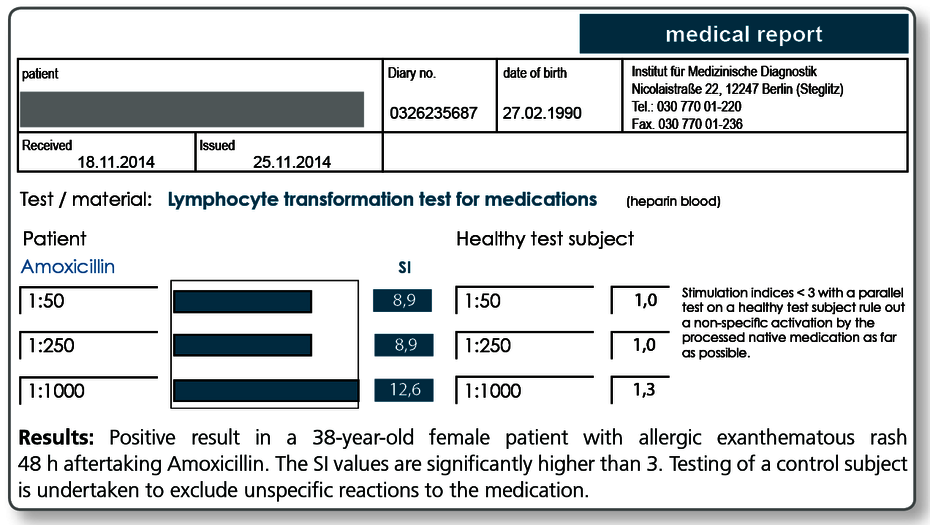
“Since skin tests and in vitro IgE testing in patients with exanthematous rashes after medication intake often do not yield any useful results, exanthematous drug eruptions are among the most common indications for requesting an LTT to fill this diagnostic gap.“
In-vitro allergy testing guideline of the German Society of Allergology and Clinical Immunology (DGAKI) in agreement with the German Dermatological Society (DDG) Journal der Deutschen Dermatologischen Gesellschaft
(2006) 4; 72-85
“The specific LTT to detect an allergic reaction to a medication can be recommended unreservedly where indicated.“
Again from the article “Quality assurance for the lymphocyte transformation test (LTT) – Addendum to the LTT paper of the committee for “Methods and Quality Assurances“ at the Robert Koch Institute, published in Bundesgesundheitsblatt 2008 RKI; 51: 1070-1076.
When should the LTT be requested?
A type IV drug allergy can be detected with the greatest accuracy using the LTT between approx. 2 weeks and approx. 3 months after the allergic manifestations have cleared.
However, positive results can still be found years later without any further (known) exposure. The fading sensitivity over time is attributed to the fact that the number of T cells circulating in the blood decreases without continued exposure. The low sensitivity during the phase of acute allergic manifestation (dermatitis, hepatitis during the first 2 weeks afterwards) is explained by the fact that the responsible T cells are staying in the affected organ (here skin or liver) and are there responsible for the local inflammation.
What sampling material is required the LTT Medications?
- Per medication, 5 mL of heparin blood is required.
- In case of 3 or 4 medications: 2 heparin Monovette blood collection tubes (20 mL heparin blood and 5 mL whole blood).
The laboratory stocks a number of medicines and active ingredients. To request current list, please call Tel. +49 30 77001-220.
Alternative, a tablet (or ampoule) of the suspected product can be send in together with the blood samples. This ensures that the administered (batchidentical) product can be tested.
Literature
- Anliker MD et al. (2003) Acute generalized exanthematous pustulosis due to sulfamethoxazol with positive lymphocyte transformation test (LTT). J Investig Allergol Clin Immunol.;13 :66-8.
- Basketter D. et al. (2005)Lymphocyte transformation test in patients with allergic contact dermatitis. Contact Dermatitis;53:1.
- Constable S et al. (2006) Systemic illness with skin eruption, fever and positive lymphocyte transformationtest in a patient on irbesartan. Br J Dermatol.;155:491-3.
- Cooper HL. Et al. (2008) Antibiotic hypersensitivity mimicking recurrent endocarditis-identifying the culprit with the in vitro lymphocyte transformation test. QJM; 101: 67-8.
- Hagemann T. et al. (2005) Positive lymphocyte transformation test in a patient with allergic contact dermatitis of the scalp after short-term use of topical minoxidil solution. Contact Dermatitis; 53: 53-5.
- Jurado-Palomo J. Et al. (2010) Use of the lymphocyte transformation test in the diagnosis of DRESS syndrome induced by ceftriaxone and piperacillin-tazobactam: two case reports. J Investig Allergol Clin Immunol.;20:433-6.
- Kano Y. (2007) Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: dependence on its timing and the type of drug eruption. Allergy; 62: 1439-44.
- Kanny G et al. (2005) T cell-mediated reactions to iodinated contrast media: evaluation by skin and lymphocyte activation tests. J Allergy Clin Immunol.;115: 179-85.
- Kardaun SH . et al. (2006) Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol.; 55: S21-3.
- Kim MH et al. (2012) A case of allopurinol-induced fixed drug eruption confirmed with a lymphocyte transformationtest. Allergy Asthma Immunol Res.;4:309-10.
- Merk HF (2005) . Lymphocyte transformation test as a diagnostic test in allergic contact dermatitis. Contact Dermatitis.; 53: 246-51.
- Merk HF. (2005) Diagnosis of drug hypersensitivity: lymphocyte transformation test and cytokines. Toxicology.; 209: 217-20. Review.
- Nyfeler B. et al. (1997) The lymphocyte transformation test for the diagnosis of drug allergy: sensitivity and specificity. Clin Exp Allergy.; 27: 175-81.
- Pichler WJ. (2005) Direct T-cell stimulations by drugs--bypassing the innate immune system. Toxicology; 209: 95-100.
- Pichler WJ. Et al. (2004) The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy.;59 :809-20. Review.
- Piñero Saavedra MC et al. (2011) Usefulness of lymphocyte activation test in atorvastatin hypersensitivity. J Investig Allergol Clin Immunol. ; 21: 574-5.
- Romano A. et al. (2011) Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 127: 67-73.
- Sachs B. et al. (2000) Drug-induced allergic cytopenia: in vitro confirmation by the lymphocyte transformationtest. Arch Intern Med. ;160: 2218-9.
- Schreiber J. et al. (1999) Lymphocyte transformation test for the evaluation of adverse effects of antituberculousdrugs. Eur J Med Res.; 25:67-71.
- Clin Exp Allergy. 1997 Feb;27(2):175-81.
- Wolf R. et al. (2005) Lymphocyte transformation test in patients with allergic contact dermatitis. Contact Dermatitis.;53 :245-50.
Dentistry / Implantology
The use of the LTT in suspected allergy to dental restorative and implant materials
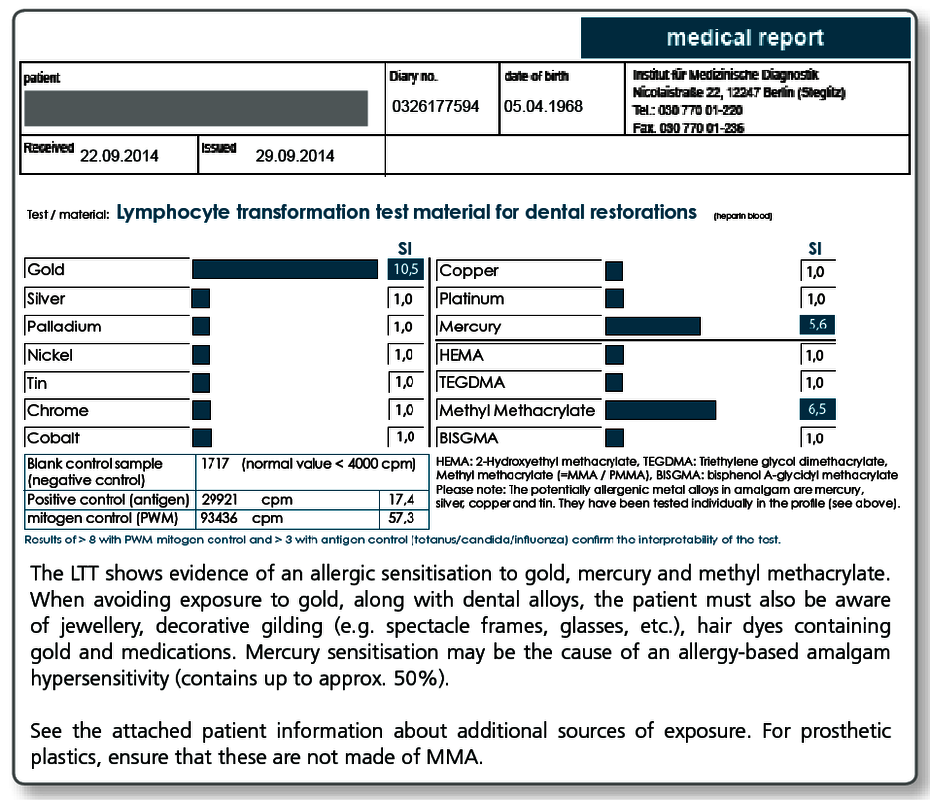
Dental restorative materials, but also incorporated materials in orthopaedic surgery (especially joint replacement implants) or trauma surgery (plates, nails, screws) contain potential allergens. This includes metals and acrylates (plastics) as well as numerous other substances used in dentistry, such as root canal filling materials, cements or ceramics. These foreign materials can act as potential allergens and cause type IV sensitisations.
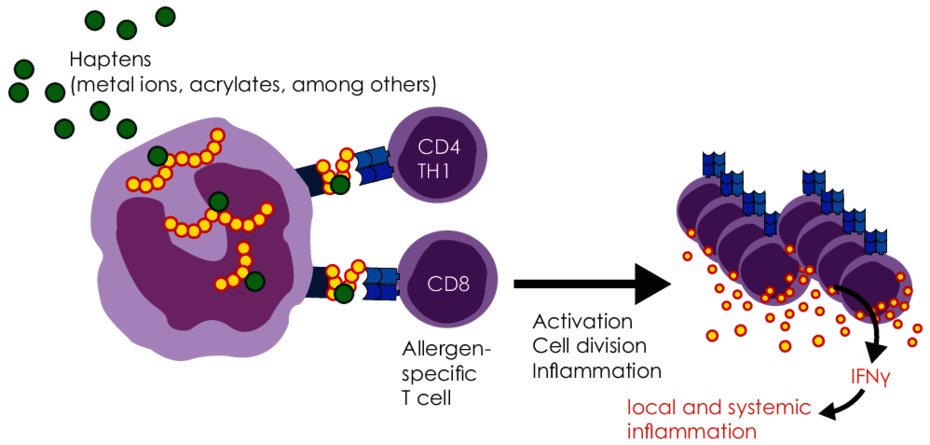
Metal ions released from alloys and acrylates present as residual monomers are haptens (half-allergens). They bind to proteins in the body and thereby change the structure of these proteins. These modified proteins appear “foreign” (allergenic) to the immune system and thus trigger an immune reaction.
Sensitisation to metals and acrylates are the result of type IV sensitisation, apart from a few exceptions. With this type of allergy, the body produces specific T cells against the corresponding allergen.
In patients who have developed a sensitisation to a metal or acrylate, the immune system responds to repeated contact with this allergen with immune activation (TH1-dominant inflammation). This can manifest in the form of local signs and symptoms, but also trigger systemic immune reactions and augment worsen inflammatory diseases.
Special clinical features of allergy-related material intolerance
Possible local oral manifestations include stomatitis, lichen ruber planus, gingivitis and periodontitis. However, these are not necessarily present as the mucous membranes of the oral cavity show little immunological reactivity. This is understandable when one considers that the oral cavity is the primary point of entrance for foreign antigens (pathogens, food, etc.).
Because of the special immunological situation in the oral mucous membrane, it is not uncommon that local symptoms, such as burning sensation on the tongue or jaw pain and toothache, do not have morphological correlates.
Since immune reactions are principally of systemic nature, numerous general symptoms can occur. These are normally not caused by the dental restorative material, but this can act as a trigger.
These unspecific symptoms include, among others:
headache, migraine, neuralgias, muscle pain, arthralgia, fibromyalgia, paraesthesia, increased tiredness, disturbed sleep, and depressive mood. It is known from clinical case studies that in sensitised patients chronic exposure to metal ions (mercury, gold, nickel, among others) can trigger autoimmunity (chronic arthritis, neurological diseases).
When should testing with the LTT be performed?
There are 2 indications for testing for materials:
- Curative question
After the introduction of foreign material into the body, symptoms appear. Here, the LTT can be used to verify or rule out that these are related to the material.
The LTT answers the question: Is it necessary to change the existing replacement material? - Preventative question
The objective here is to rule out that at the time of introduction of new materials into the body, the patient has an allergic sensitisation to a contact allergen contained in these materials.
The preventive LTT answers the question: What materials can be used or should not be used?
| Please note: Preventive testing should only be perform using the LTT and not with the epicutaneous test, because with latter the patient is exposed to a potential allergen (in the skin) and this by itself can result in (iatrogenic) sensitisation. For this reason, the epicutaneous test guideline of the German Contact Allergy Groupdoes not recommend preventive testing. |
Which materials can be tested for using the LTT?
In principle, the LTT can be used to test for almost any material and material component.
For native materials, a cytotoxic effect or unspecific activating properties have to be ruled out in the laboratory in advance or through parallel testing of control subjects.
For common questions, profiles were developed to test for the known sensitising individual allergens in a standardised way.
LTT profiles
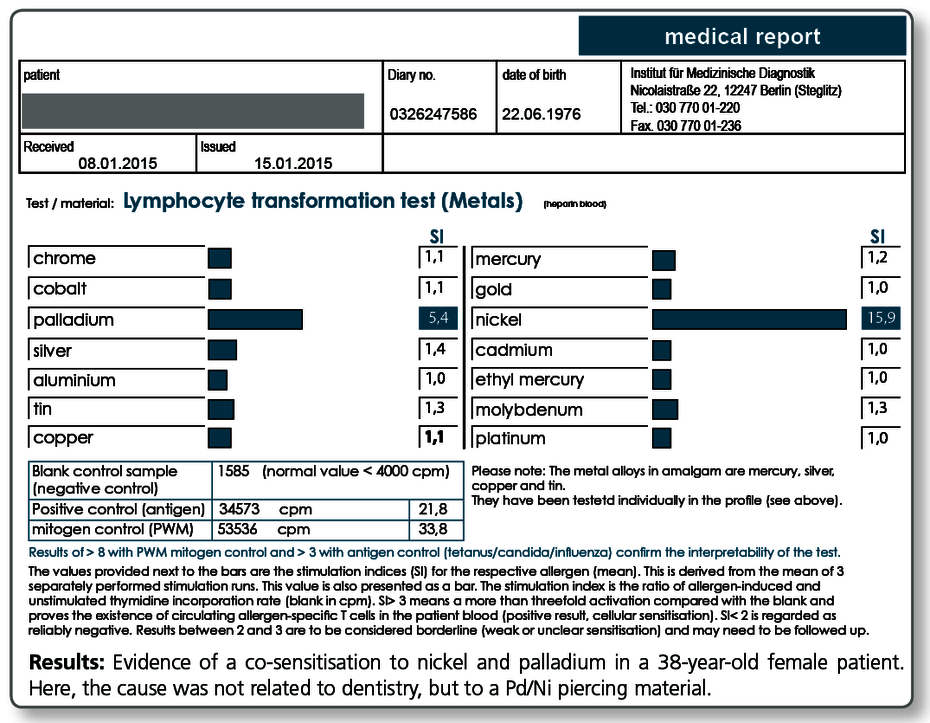
| LTT Metals | 14 standard metals: mercury, copper, silver, tin, ethylmercury, gold, nickel, palladium, chrome, cobalt, molybdenum, aluminium, platinum, cadmium |
| LTT Plastics | TEGDMA, BISGMA, BISDMA, HEMA, methyl methacrylate (MMA), diurethane dimethacrylate (DUDMC), ethylene glycol dimethacrylate, butanediol 1,4-methacrylate, hydroquinone, N,N-dimethyl-4-toluidine, benzoyl peroxide, formaldehyde, phthalate, camphorquinone |
| LTT combination profile (dental check) | Metals: gold, nickel, palladium, chromium, cobalt, platinum, mercury, copper, silver, tin Plastics: methyl methacrylate (MMA), hydroxyethyl methacrylate (HEMA), TEGDMA, BISGMA |
| LTT Gold | Alloys gold + alloy components: gold, silver, platinum, copper, palladium, tin, gallium, indium, iridium, rhodium, tantalum, ruthenium |
| LTT Amalgams | amalgam components and organic mercury compounds: mercury, copper, silver, tin, ethyl mercury, phenyl mercury, methyl mercury |
| LTT Root Canal Filling Materials | raw gutta-percha, Balsam of Peru, eugenol, PDMS, silicone oil, bismuth oxide, turpentine oil, colophony, triethanolamine, peanut oil, paraformaldehyde, bisphenol A, epichlorohydrin |
| LTT Ceramic and Cements | vanadium, aluminium, titanium, cobalt, chromium, barium, silicon, cerium, boron, manganese, antimony, phosphate cement (Harvard), glass ionomer base cement (Ketac-Bond) |
| LTT-Titanium Materials | Titanium + Alloy Components: titanium dioxide, nickel, vanadium, aluminium |
| LTT Endoprosthetics | chromium, cobalt, molybdenum, nickel, titanium, vanadium, niobium, aluminium, zirconium (IV) oxide, methyl methacrylate, N,N-dimethyl-p-toluidine, benzoyl peroxide, hydroquinone, gentamicin |
| LTT Native Material | testing for native materials submitted together with blood samples |
For titanium intolerance testing, please refer here.
LTT Metals
LTT Plastics
Is it also possible to tested for individual materials?
With the LTT Native Material, there is also the option of testing materials which are submitted by the physician, dentist or the dental lab together with the blood sample to the laboratory. This procedure has proven to be successful particularly with complex materials like cements, composites, prosthesis materials, bone graft substitute materials, root canal filling materials (also pins) as well as intraoral harvested metal and plastic chips and shavings.
If materials have tested negative in the LTT, it is ruled out that there is sensitisation to substances contained in these materials whether they are declared or not. Materials which have tested positive in the LTT should under no circumstances be placed in the patient's body, because the patient has a type IV sensitisation to at least one of the components.
note: A selection of native materials (commonly used composites etc.) is available in the laboratory. Please call us under +49 30 77001-220 and we will send you this list per fax.
LTT Native Materials
Are any cost-effective combined profiles available?
A combined profile (Dental-Check) was compiled in particular for preventive testing in dentistry which covers the most important metals and acrylates. Although this profile is not comprehensive, it covers the most important contact allergens, i.e. those allergens in the test profiles LTT Metals and LTT Plastics which most frequently tested positive in the past.
| Metals | gold, silver, palladium, copper, tin, cobalt, chromium, nickel, platinum, and mercury |
| Acrylates | hydroxyethyl methacrylate (HEMA), methyl methacrylate (MMA), triethylene glycol dimethacrylate (TEGDMA), BISGMA |
The LTT Dental Check thus includes the problem areas:
- gold alloys (Au, Pt, Pd, Ag, Cu, Sn)
- NEM alloys (Cr, Co, Ni)
- amalgam (Hg, Ag, Sn, Cu)
- acrylate containing composites and cements (HEMA, TEGDMA, BISGMA)
- acrylic resins for prostheses (MMA)
LTT Dental Check
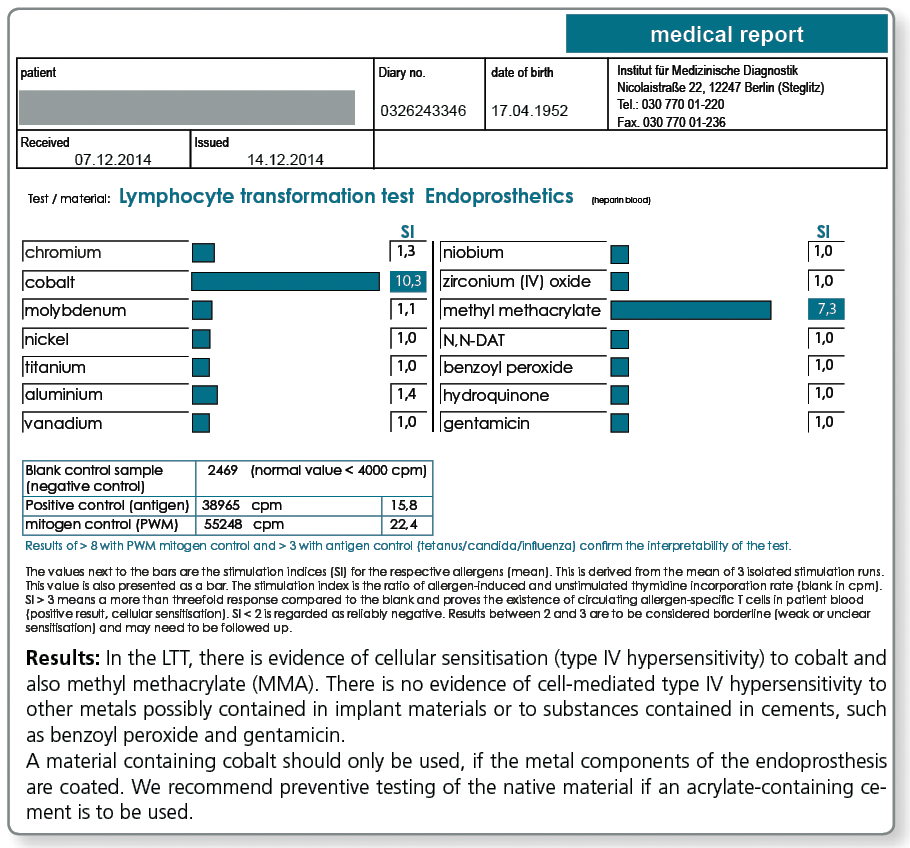
Is there a special profile available for testing ahead of joint replacement surgery?
Especially for preventive testing a combined profile was compiled, which includes, besides the metals potentially contained in implants, the components of cements. Among these components are acrylates, the polymerisation initiators benzoyl peroxide and hydroquinone, as well as gentamicin which is found in most cements.
LTT Endoprosthetics
What about titanium?
Attention:A negative LTT result for titanium rules out only an allergic sensitisation which is very rare. To find out whether the patient has an increased propensity for inflammation on exposure to titanium oxide particles, especially in preventive questions, it is necessary to investigate any genetic predisposition for inflammation (TNF-α, IL-1α, IL-1β and IL-1RA) and to perform the titanium stimulation test. |
Please request the “Titanium Intolerance” diagnostic information sheet from our laboratory.
What are the consequences of a positive LTT result?
A positive test result in the LTT (but also in skin testing) indicates an existing sensitisation to the respective allergen. The material that tested positive should not be used in future treatments.
However, no allergy test can prove that there is a causal link to existing symptoms. Thus, with a proven sensitisation it must be carefully considered whether the respective problem material should be removed and replaced with a different material.
Here, the severity of the symptoms is critical, by no means a positive test result alone. Other sources of exposure should be eliminated primarily or at the same time (detailed information to other sources of exposure is available with every positive LTT result).
When is the Multi-Element Analysis/MEA of the metals advisable?
In particular with dentistry-related queries, sometimes the question arises whether a confirmed sensitisation to a certain metal has therapeutic consequences.
This is only the case when the patient has been exposed to the respective metal through existing restorative dental work. In some cases this is not clear, for example, when it is not known whether older existing gold alloys contain copper, silver or palladium or whether there is a potential exposure to nickel or chromium from a (usually not declared) point of soldering in the dental work.
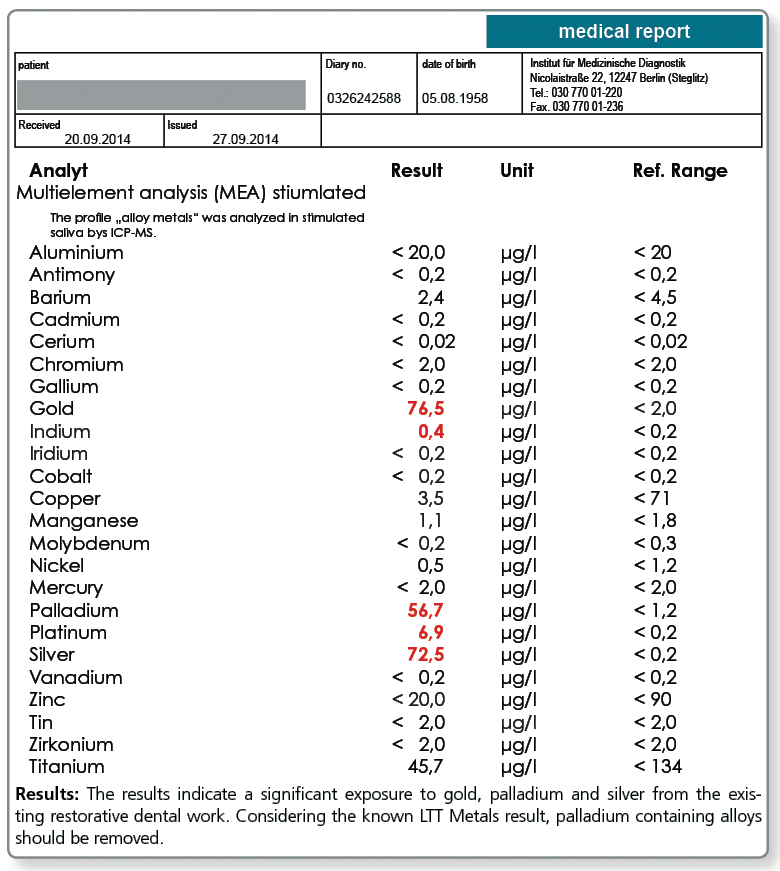
For these cases, the multi-element analysis in the saliva can be a useful addition to the diagnostic work-up.
If the saliva test (morning saliva or chewing gum test) shows detectable values for the metal that tested positive in the LTT, the source of the substance has to be investigated and the material has to be removed, if possible. If a patient has a type IV sensitisation to a metal, even mild elevations are of relevance which is in contrast to the toxicological perspective where only significantly increased metal levels in saliva are regarded as clinically important.
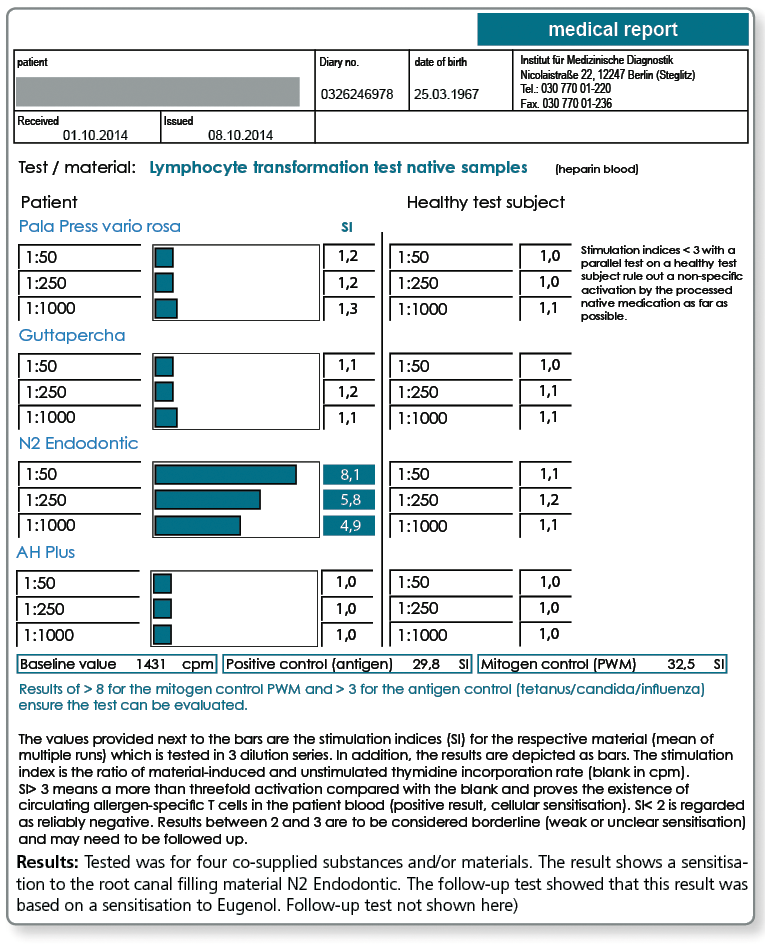
Below a sample case report of a 44-year-old female patient who tested positive in the LTT for a sensitisation to aluminium, nickel and palladium. The multi-element analysis of her morning saliva identifies her existing restorative dental work as the source of her significant exposure to gold, silver and also palladium. In so far, a removal of the apparently palladium-containing gold alloys is indicated even though there is no allergic sensitisation to gold itself.
The patient is not exposed to aluminium and nickel, at least not by restorative dental work. Here, the source of exposure must be searched elsewhere (food, toner exposure etc.).
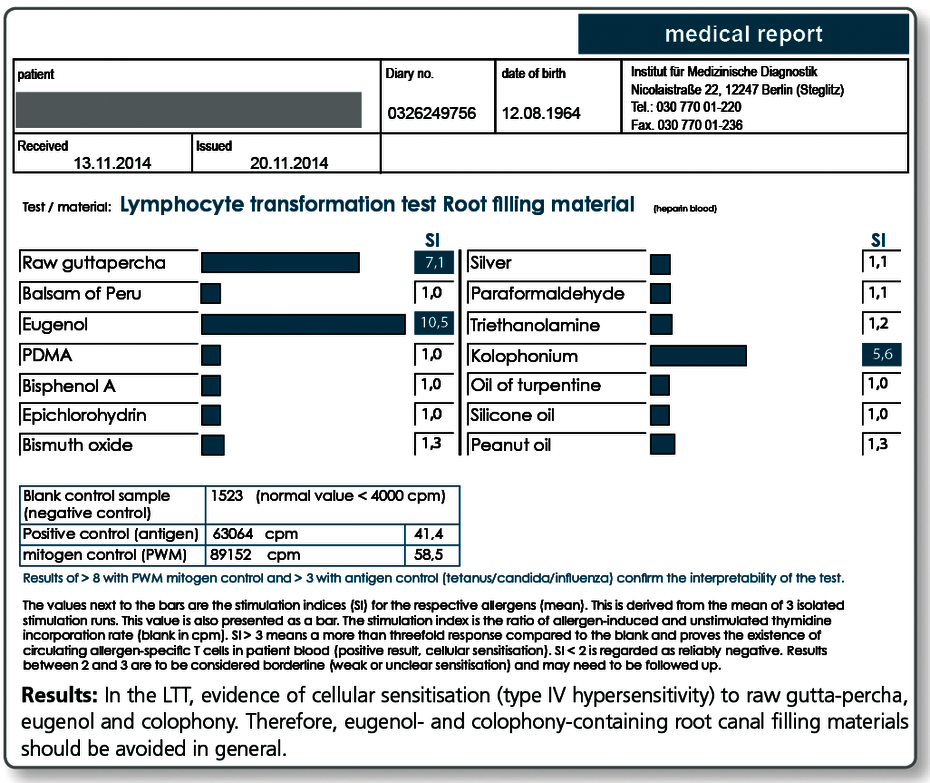
Are there any allergies against root canal filling materials?
Yes, gutta-percha and also the various available root canal sealers on the market often contain one or more substances that give rise to concern. These include very potent allergens such as Balsam of Peru, bisphenol A, paraformaldehyde and silicone, peanut or turpentine oil.
For curative, but even more so for preventive queries, the profile LTT Root Canal Filling Materials is indicated. Apart from gutta-percha, this profile contains those ingredients of commonly used root canal sealers that are known to have caused problems.
In case of a positive test result, a material must be selected which does not contain the respective substance.
Allergy testing for gutta-percha uses natural raw gutta-percha. Especially with preventive queries it can be useful to test the specific gutta-percha product to be used, because, for example, synthetically produced gutta-percha or processed natural raw materials do not necessarily contain any longer all allergen components of the raw gutta-percha.
Effector cell typing is never a substitute for the LTT in preventive testing
In individual cases with proven sensitisation in the LTT and uncertain clinical significance, it is possible to confirm or invalidate the causal relationship between sensitisation and clinical manifestation by subsequently performing an effector cell typing.
In effector cell typing, the effector cells involved in the immune reaction are closer characterised (typed) by the allergen-stimulating cytokine reactions. Differentiation occurs into IFN-γ secreting TH1 effector cells, into IL-10 releasing regulatory T cells and into memory cells without current effector function which (only) release IL-2. Especially if no previous LTT Result is available, the test profile is complemented by IL-2 and TNF-α.
However, effector cell typing is no alternative to LTT, especially in preventive testing, because it cannot detect present latent allergies with certainty. In fact, there are memory lymphocytes which do not release cytokines.
A negative effector cell typing result should not lead to the decision to introduce the respective material into the body, because with permanent exposure to the respective allergen it is likely that the effector reaction develops into a cytotoxic TH1 immune reaction.
Please contact us for further information about effector cell typing.
Is the LTT a validated laboratory method?
At present, the LTT is the only laboratory method to test for specific cellular sensitization. By further development in cell culture technique and analytical methods the test has emerged to a reproducible and highly sensitive method for medical diagnostic testing.
A positive reaction in the LTT indicates the presence of allergen-specific lymphocytes (memory cells) in the patient’s blood.
Since 2004, the LTT has been accredited by DAkkS according to DIN EN ISO 17025 and its sensitivity and specificity are monitored by ring and comparative testing.
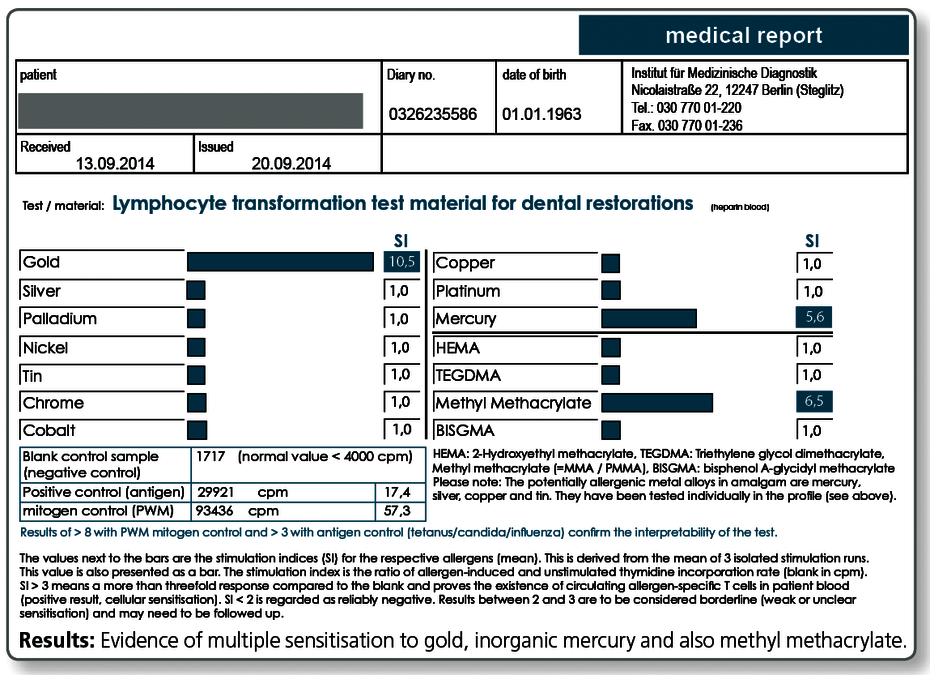
Although today the LTT is generally professionally recognized as a method to detect sensitisation to medications and beryllium, the LTT’s validity in metal or plastics allergies is still discussed controversially.
This is insofar surprising, as the methodology does not differ between the various areas of use.
The reason for the differentiated view is unfortunately rather related to differences in the perception of the problem areas drug allergy and material intolerance.
Whereas drug allergy is not considered controversial, very conservative allergists see the metal and acrylate allergies as rare conditions and think that the reported symptoms are rather fully or partially of psychosomatic origin.
In this point in particular, the LTT has provided valuable services by helping to provide objective parameters in relation to the symptoms.
Literature
- von Muris J. et al.(2009) Reactivity to sodium tetrachloropalladate (Na2[PdCl4]) compared to PdCl2 andNiCl2 in lymphocyte proliferation tests. Allergy.; 64: 1152-6.
- Thomas P. et al. (2009) Increased metal allergy in patients with failed metal-on-metal hip arthroplasty and peri-implant T-lymphocytic inflammation. Allergy.; 64: 1157-65.
- Vamnes JS et. al.(1999) In vitro lymphocyte reactivity to gold compounds in the diagnosis of contact hypersensitivity. Contact Dermatitis.;41:156-60.
- Thomas P et al. (2006) Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis.;55: 199-202.
- Rasanen L. Et al. (1992) Diagnostic value of the lymphocyte proliferation test in nickel contact allergy and provocation in occupational coin dermatitis. Contact Dermatitis.; 27: 250-4.
- von Baehr V et al. (2001) Improving the in vitro antigen specific T cell proliferation assay: the use of interferon-alpha to elicit antigen specific stimulation and decrease bystander proliferation. J Immunol Methods.;251: 63-71.
- Bartram F. et al. (2006) Stellungnahme des Deutschen Berufsverband der Umweltmediziner zur Bedeutung von Epikutantest und Lymphozytentransformationstest für die Diagnostik von Typ IV-Sensibilisierungen. Journal of Laboratory Medicine ; 30 : 101-106).
- Everness KM. Et al. (1990) The discrimination between nickel-sensitive and non-nickel-sensitive subjects by an in vitro lymphocyte transformation test. Br J Dermatol.;122: 293-8.
- Lebeau J. (2007) Use of the Beryllium Lymphocyte Proliferation Test (BeLPT) for screening. J Occup Environ Med.;49: 357-8.
- Cederbrant K. et al. (1997) In vitro lymphocyte proliferation as compared to patch test using gold, palladium and nickel. Int Arch Allergy Immunol.;112: 212-17.
- Rustemeyer T. et al. (2004) Analysis of effector and regulatory immune reactivity to nickel. Clin Exp Allergy; 34: 1458-66.
- Hallab NJ. et al. (2004) Lymphocyte transformation testing for quantifying metal-implant-related hyper-sensitivity responses. Dermatitis.;15: 82-90.
- Hallab NJ. et al. (2005) Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. ;23: 384-91.
- Stange AW. Et al. (2004) , Furman FJ, Hilmas DE. The beryllium lymphocyte proliferation test: Relevant issues in beryllium health surveillance. Am J Ind Med.;46: 453-62.
- Milovanova TN. et al. (2007) Comparative analysis between CFSE flow cytometric and tritiated thymidine incorporation tests for beryllium sensitivity. Cytometry B Clin Cytom.;72: 265-75.
- Moneret-Vautrin D.A. (2004) Allergy to nickel in dental alloys. Europ. Annals of Allergy and Clin Immunol; 36: 311-2.
- piewak R, Moed H, et al. (2007) Allergic contact dermatitis to nickel: modified in vitro test protocols for better detection of allergen-specific response. Contact Dermatitis.; 56: 63-9.
- Valentine-Thon E. et al. (2006) LTT-MELISA(R) is clinically relevant for detecting and monitoring metal sensitivity. Neuro Endocrinol Lett. ;27: 17-24.
- Valentine-Thon E et al. (2003) Validity of MELISA for metal sensitivity testing. Neuro Endocrinol Lett.;24 : 57-64.
- Lindemann M. et al. (2008) Detection of chromium allergy by cellular in vitro methods. Clin Exp Allergy.; 38: 1468-75.
- Räsänen L et al. (1991) Lymphocyte proliferation test as a diagnostic aid in chromium contact sensitivity. Contact Dermatitis.; 25: 25-9.
- Deubner DC et al. (2001) Variability, predictive value, and uses of the beryllium blood lymphocyte proliferation test (BLPT). Appl Occup Environ Hyg.;;16: 521-6.
- Minang JT et al. (2006) Nickel, cobalt, chromium, palladium and gold induce a mixed Th1- and Th2-type cytokine response in vitro in subjects with contact allergy to the respective metals. Clin Exp Immunol.; 146: 417-26.
- Stange AW. Et al. (2004) The beryllium lymphocyte proliferation test: Relevant issues in beryllium health surveillance. Am J Ind Med.;46: 453-62.
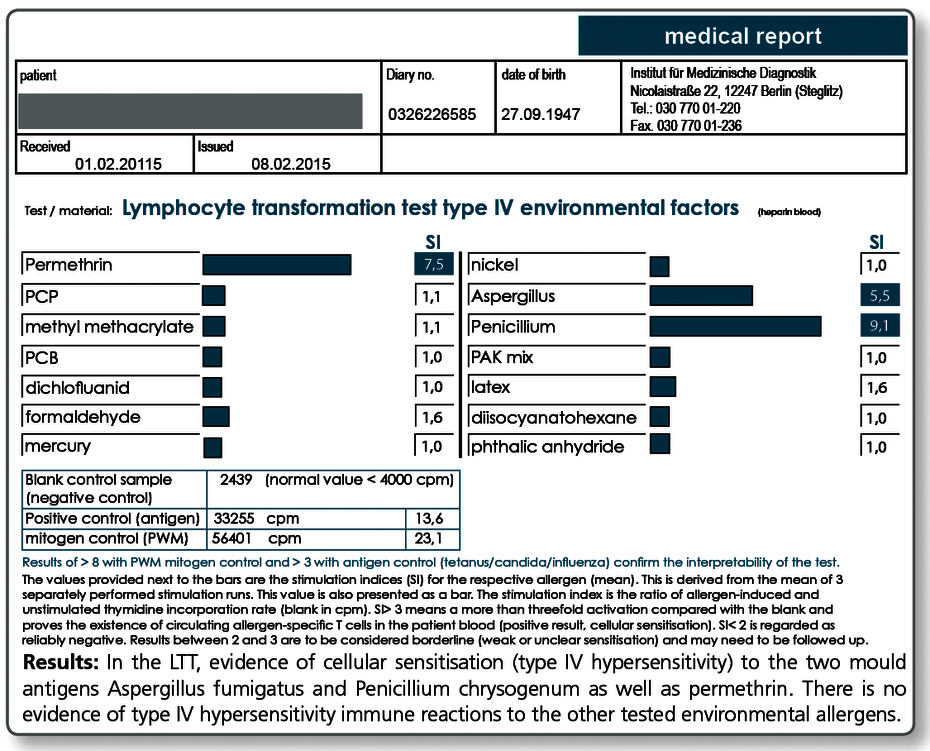
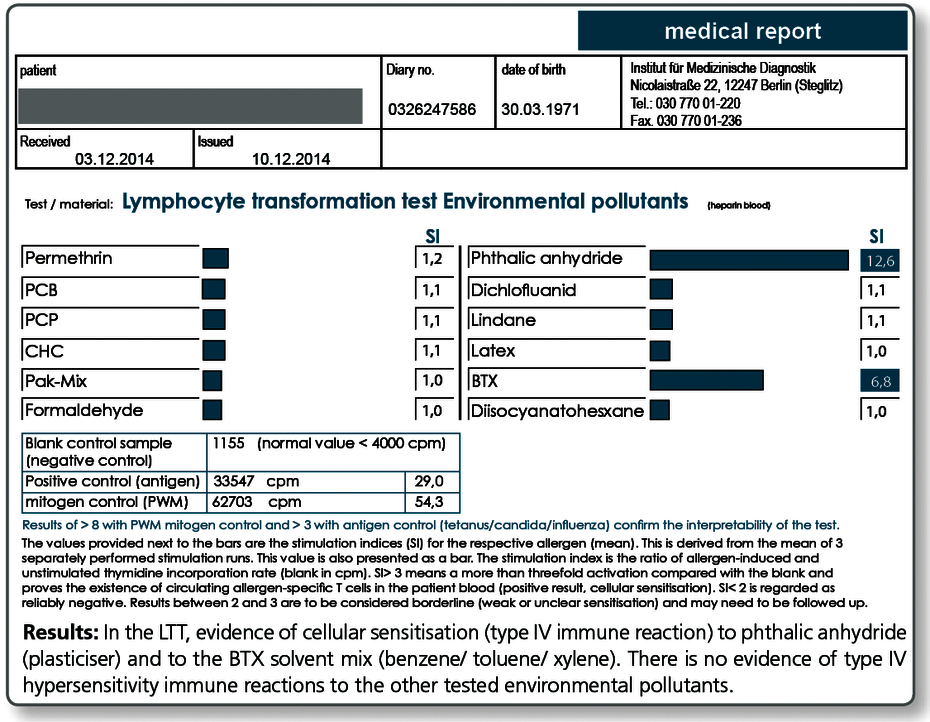
Environmental pollutants
Use of the LTT to detect individual sensitisations to environmental pollutants
Due to their hapten effects, almost all chemical substances can cause a type IV allergy similar to metals or medications. The increasing importance of xenobiotics in our environment explains the growing number of immunological sensitisations.
Pollutants with sensitisation properties include among others
- phthalates (plasticisers)
- wood preservatives
- fungicides
- herbicides
- insecticides
- solvents
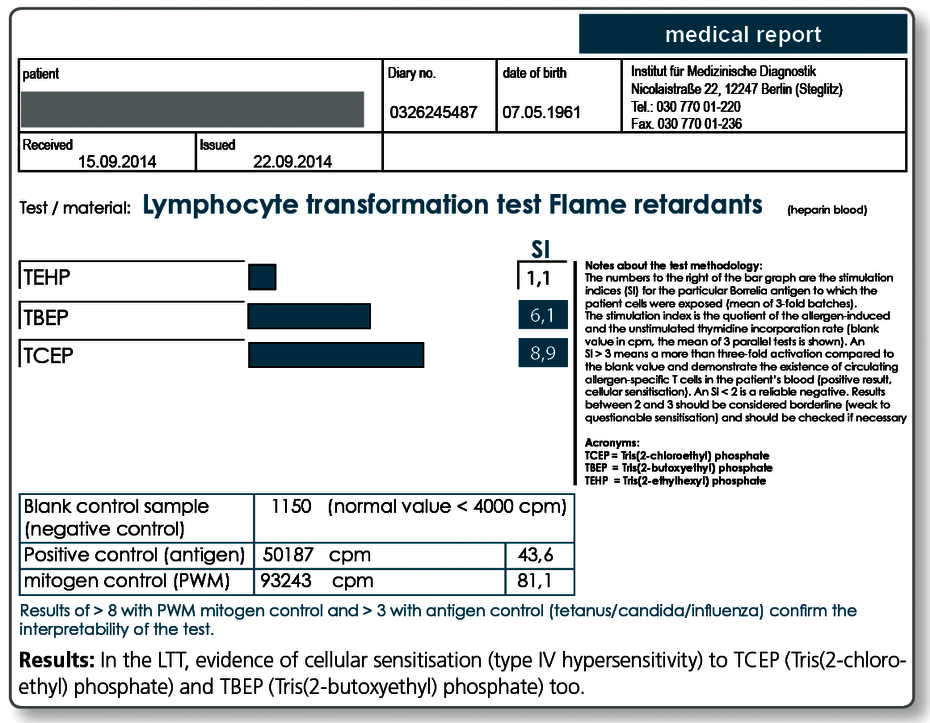
- flame retardants
With these substances, in-vitro testing using the LTT should always be preferred to the epicutaneous test because:
- many of the mentioned substances have skin-irritating properties.
- it cannot be ruled out that epicutaneous testing itself can cause a sensitisation to the pollutant.
- a carcinogenic effect to the skin is possible
- in the interpretation of positive test reactions it cannot be distinguished between allergy- and irritation-triggered skin reactions.
With the LTT, a great number of pollutants can be tested in a standardized way. To make lab requests easier, the most important allergens have been compiled into profiles which are content-wise partially overlapping.
LTT MCS Environmental Factors:
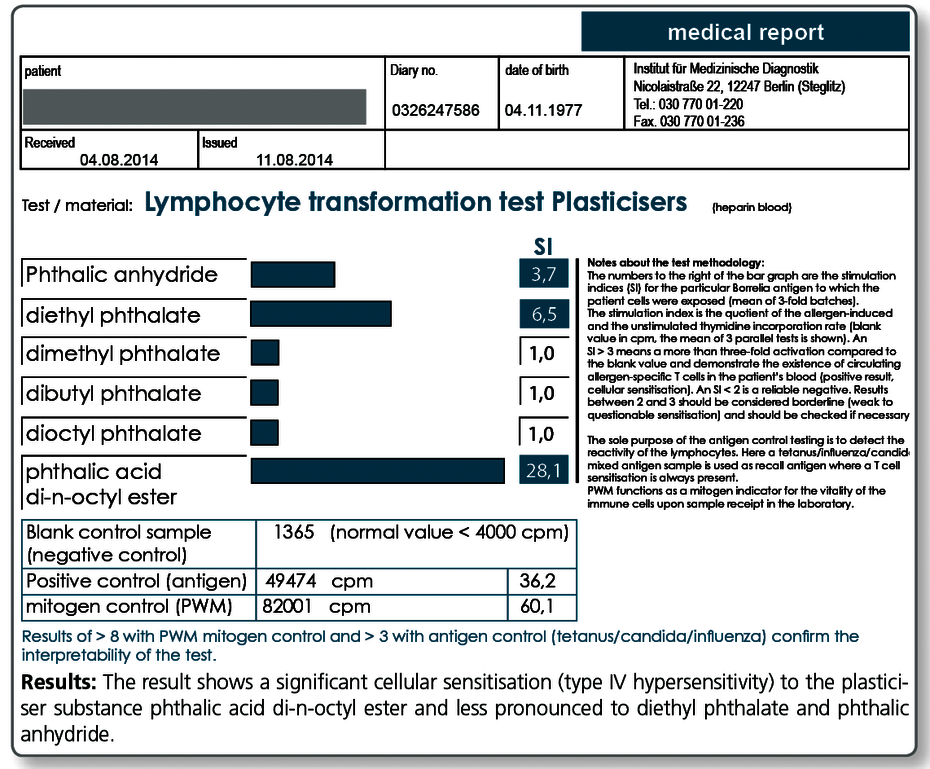
Tests are performed for nickel, mercury, latex, PCP, PCB, permethrin, formaldehyde, methyl methacrylate, Aspergillus fumigatus, Penicillium chrysogenum, phthalic anhydride, dichlofluanid, PAK mix, 1,6-diisocyanatohexane
LTT Environmental Factors
LTT Environmental Pollutants
LTT Flame Retardants
LTT Plasticisers
Literature
- Sieben S. et al. (2001) Characterization of T cell responses to fragrances. Toxicol Appl Pharmacol. ;172: 172-8.
- Wolf R. et al. (2005) Lymphocyte transformation test in patients with allergic contact dermatitis. Contact Dermatitis ;53: 245.
- Kusaka Y. et al. (1991) Lymphocyte transformation test with nickel in hard metal asthma: another sensitizing component of hard metal. Ind Health.; 29: 153-60.
- Li Q, Wang ZY, et al. (1995) Evaluation of contact sensitivity to formaldehyde and tetramethylthiuram monosulfide using a modified lymphocyte transformation test. Toxicology.; 104: 17-23.
- van Bever HP. Et al. (1993) Lymphocyte transformation test with house dust mite (Dermatophagoides pteronyssinus) in normal children, asthmatic children and asthmatic children receiving hyposensitization. Clin Exp Allergy.;23: 661-8.
- Merk K.H.(2994) Allergische Berufsdermatosen, Stellungnahme zur In vitro-Diagnostik; Hautarzt 55;31-34.
- von Baehr V. et al. (2005) Allergoid-specific T-cell reaction as a measure of the immunological response to specific immunotherapy (SIT) with a Th1-adjuvanted allergy vaccine. J Investig Allergol Clin Immunol.; 15: 234-41.
- Sasaki K. et al. (1992) Lymphocyte transformation test for house dust mite in atopic dermatitis: relationship between mite antigens for type I and type IV allergy. Acta Derm Venereol ; 176: 49-53.
- Berger P. et. Al. (1991) The lymphocyte transformation test with type II collagen as a diagnostic tool of autoimmune sensorineural hearing loss. Laryngoscope. ; 101: 895-9.
- Li Q. et al. (1996) Evaluation of cross-sensitization among dye-intermediate agents using a modified lymphocyte transformation test. Arch Toxicol. ;70: 414-9.
- Kimber I. et al. (1991) Lymphocyte transformation and thiuram sensitization. Contact Dermatitis.;24: 164-71.
- Kanerva L. et al. (1988) Sensitization to patch test acrylates. Contact Dermatitis.;18: 10-5.
Moulds
Use of the LTT to detect type IV sensitisation to moulds
Moulds grow in all moist and warm locations or where plant materials are present that they can use as a source of energy. Unlike plants, moulds do not have any chlorophyll and therefore cannot obtain their energy from sunlight.
Moulds are made up of a cellular mesh known as the hyphae, which first colonise the surface of the materials and later penetrate deeper. After longer periods of colonisation, the ‘energy sources’ they live off are destroyed: building materials and wallpapers are broken down, wood and paper crumbles, plaster and paints peel off.
Another typical source of energy is potting mixture or the dead parts of pot plants as well as food items.
Like all living organisms, fungi also need water to grow. However, if water is missing the fungi does not die, but instead forms so-called “persistent cells”. These enable the fungi to survive “periods of stress”. With the return of more favourable growth conditions (humidity), the fungi start growing again. The spores (seeds), which are used for reproduction are microscopically small. They float in the air and are either breathed in or ingested with food. They can cause allergies if an individual is sensitised to them.
Many of the approximately 10,000 known mould species are exceptionally potent allergens, although only a few of these are found indoors or in the immediate living environment of patients (garden compost, organic waste, etc.).
The most important fungi present in living environments are the genera Alternaria, Aspergillus, Cladosporium, Stachybotrys, and Penicillium.
If there is an allergic sensitisation (high stimulation index in the LTT or detection of IgE antibodies) to one or more of these moulds, it is important to avoid exposure. The general therapeutic management includes first and foremost avoiding contact with the allergen which requires stringent removal of all allergen sources.
The Profile LTT Moulds comprises:
- Alternaria alternata (former name A. tenius)
- Aspergillus fumigatus
- Penicillium chrysogenum (former name P. notatum)
- Trichophyton mentagrophytes
- Cladosporum herbarum
- Mucor mucedo
- Rhizopus nigricans
- Stachybotris spp.
- Botrytis cinerea
- Candida albicans (yeast)
LTT - food
Use of the LTT to detect type IV sensitisations to food
Without any doubt, food intolerances have steadily increased over the last 20 years. Information about the frequency of those affected in Germany varies between 11 and 41%.
The term food intolerance is an umbrella term for all unwanted symptoms or diseases associated with the consumption of specific food items.
Besides toxic reactions (food poisoning etc.) and structure-related problems (stomach resection, diverticulum, pancreatitis etc.), the food intolerances are divided in two reaction types: IMMUNOLOGICAL and NON-IMMUNOLOGICAL.
As shown in the following figure, type IV allergies detected with the lymphocyte transformation test belong to the immune-mediated food intolerances and should be distinguished from type I allergies, the pseudo-allergies and also from celiac disease.
| IMMUNOLOGICAL | |
|---|---|
| ALLERGIES | TYP I (immediate type) TYP IV (delayed type) Cross allergies to inhalation allergens |
| AUTOIMMUNE DISEASE | Coeliac disease |
| PSEUDO-ALLERGY | Food additives |
| NON-MMUNOLOGICAL | |
|---|---|
| ENZYME DEFECTS | Lactose intolerance Fructose intolerance Histamine intolerance |
| FRUCTOSE malabsorption | |
| Impaired PHYTOSTEROL absorption | |
The classical food allergy is a type I reaction, mediated by specific IgE antibodies.
However, particularly among adults other intolerance reactions may also occur. Some authors report up to 50% IgE-independent mechanisms. Clinical reaction patterns indicative of intolerances of the delayed type (allergy of type IV) are increasing seen in adults.
Food intolerance of the delayed type IV should always be taken into consideration if:
- the reaction occurs only after some hours and lasts for several days or longer.
- eczematous skin changes, gastrointestinal symptoms, myalgia and arthritic symptoms are the main complaints.
The lymphocyte transformation test (LTT) as a diagnostic tool for type IV sensitisation to food has become an established and validated method of the modern laboratory diagnostics.
The use of the LTT to test for food proteins became possible with the utilisation of LPS-cleaned allergens in the diagnostic testing and methodological changes to the LTT which increased its specificity und sensitivity (see von Baehr et al. Journal Immunol. Methods 2001; 151: 63-71).
The LTT is used in food intolerances in particular when no corresponding specific IgE antibody have been detected in the CAP test (a common situation in adults) and when non-allergic food intolerances, such as coeliac disease, lactose intolerance or histamine intolerance, can be ruled out by the clinical presentation or laboratory diagnostics.
There are 2 test profiles available:
- the LTT Food Screen 75 or alternatively
- the LTT TOP25 Food
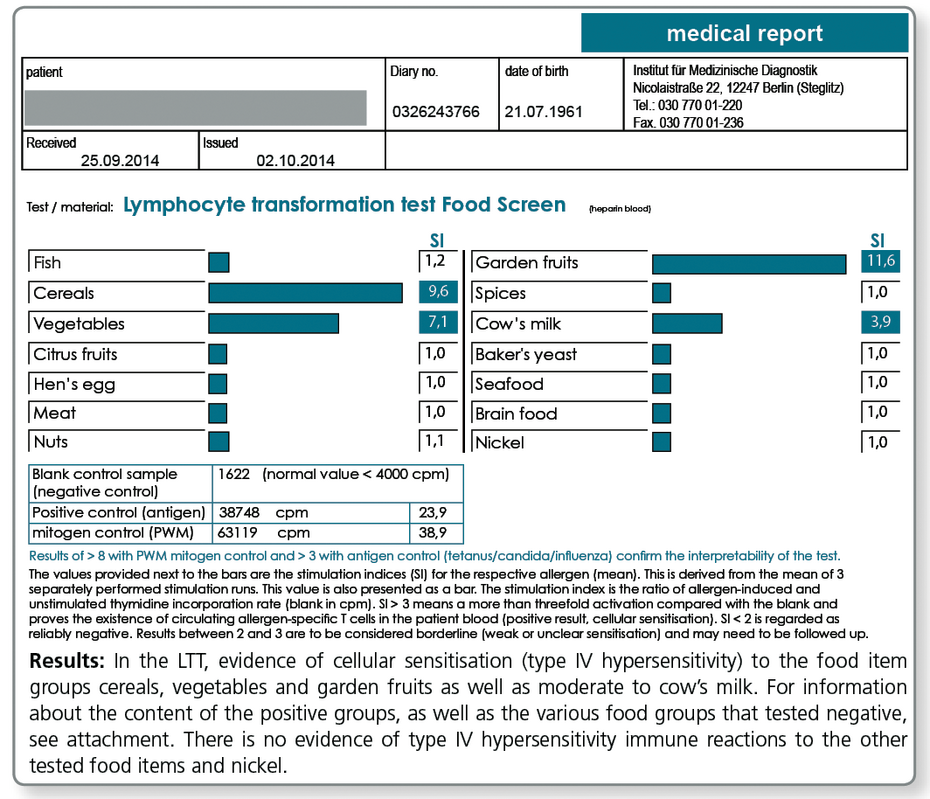
In LTT Food Screen 74 food items and in addition the potential contact allergen nickel, frequently present in food items as a contaminant, can be studied in one test run.
The food test is performed in groups of 2 to 9 allergens.
| Ingredients of the Food Test Groups in the LTT Food Screen | |
|---|---|
| Meat | pig, chicken, beef, turkey, mutton (lamb), goose, duck |
| Fish | trout, eel, halibut, salmon, cod, carp, tuna, herring |
| Seafood | lobster, shrimp, crayfish, sole |
| Cereals | wheat, barley, rye, oat, rice, spelt, soy, corn |
| Brain food | Black tea, hops, cocoa beans, coffee beans, brewer's yeast |
| Milk | α-lactalbumine, casein, β-lactoglobulin, BSA (bovine serum albumin) |
| Nuts | peanut, walnut, hazelnut, Brazil nut, pistachio, almond, cashew nut |
| Garden fruits | apple, pear, strawberry, peach, grapes |
| Citrus fruits | Orange, grapefruit, lemon, pineapple, mandarin, avocado, kiwi fruit, banana |
| Spices | mugwort, garlic, coriander, paprika, pepper, vanilla, cinnamon, aniseed |
| Vegetables | pea, carrot, potato, cauliflower, celery, asparagus, spinach, tomato, onion |
| Hen’s egg | egg yolk, egg white |
| Yeast | baker's yeast |
| Nickel | as a frequent metal ion pollutant |
LTT 75
LTT Group
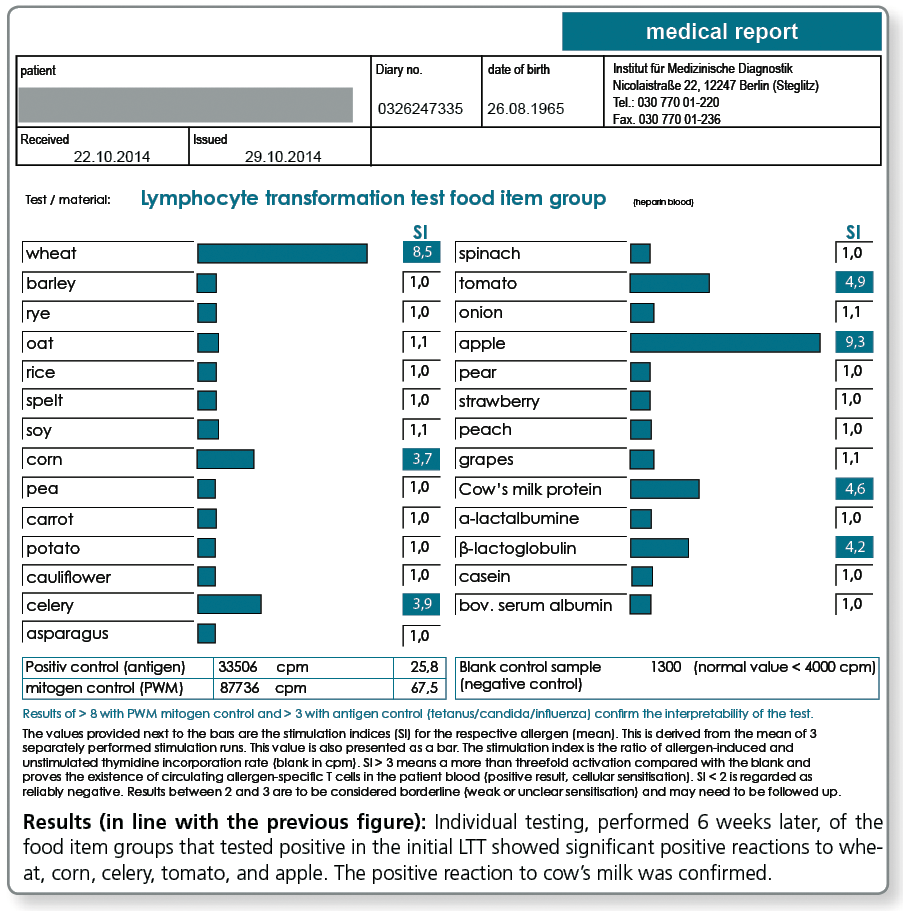
The LTT Food Screen has the advantage that it covers with the tested food items approximately 98% of all known type IV food intolerances. A disadvantage is that the testing in this screening test is performed in groups (e.g. cereals, nuts etc.) and a positive result for one group needs a follow-up testing to specify the single allergens of that group. This requires a second blood collection.
In the LTT TOP 25 food items, the insights from the LTT Food Screen 75 gained over many years were used to select the 25 of allergens which most frequently tested positive. In the LTT, these are tested as single allergens already in the initial test.
The advantage of this profile is that it does not require follow-up testing for positive screening results, eliminating the need for a second blood collection and any costs for follow-up testing of positive groups. According to statistical data, approx. 92% of the type IV sensitisations to food are recognised.
The food items included in the LTT Food TOP 25 are shown in the following results report.
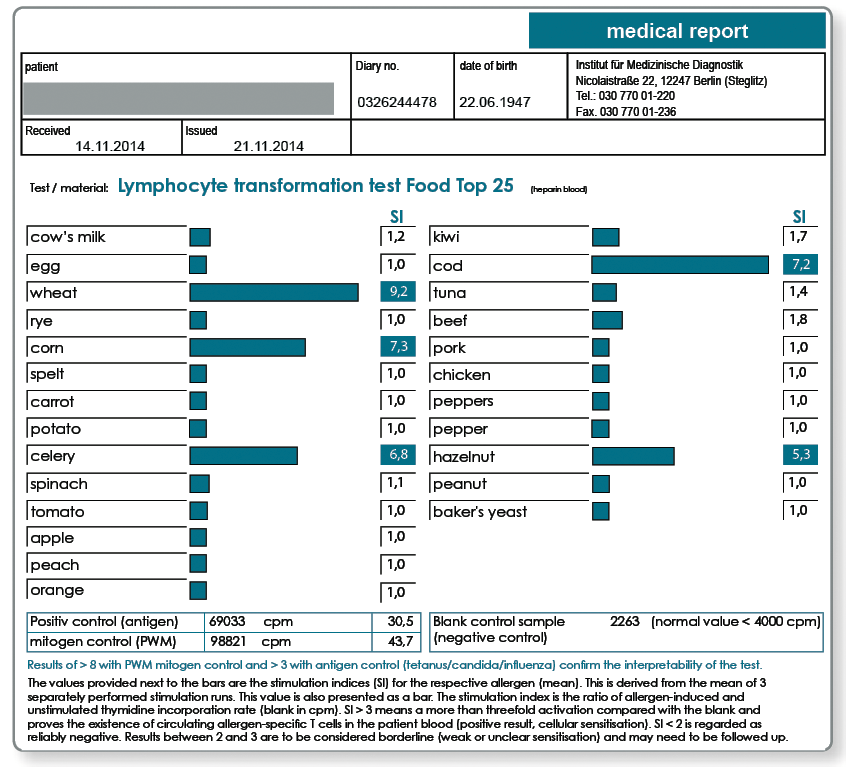
LTT TOP 25
What material is required for blood collection?
- LTT Food Screen 75: 20 mL heparin blood and 5 mL whole blood
- LTT to individual food groups (according to result of the LTT Food Screen): per group 20 mL heparin blood and once 5 mL whole blood
- LTT Food Item TOP 25: 30 ml heparin blood and 5 mL whole blood
Please request the appropriate collection materials (LTT blood collection sets) from the laboratory free of charge.
No more than 24 hours should elapse between the time the blood is collected and the receipt of the sample in the laboratory. During this time the blood samples should be stored at room temperature. Blood samples for cellular function tests should not be stored in the fridge!
Food additives
Special case: Food additives and colouring agents
The allergy testing for food additives is not performed using the LTT, because the basophile degranulation test (BDT) has been established as the more appropriate method.
The Profile for Food Additives includes:
- Food colouring agents mixture I
(amaranth, azorubine, quinoline yellow, cochineal red A, yellow-orange - Food colouring agents mixture II
(erythrosine, patent blue, indigo carmine, brilliant black) - Food additives I
(tartrazine, sodium benzoate, sodium nitrite, potassium metabisulphite, sodium salicylate) - Food additives II
(iron oxide, benzoic acid, glutamate, propyl-p-hydroxybenzoate)
If the group screening yields a positive result, the substances contained in the group can then be individually tested, as with the LTT. However, this requires a new blood sample! If a particular substance is suspected, individual testing can be performed first (e.g., glutamate with suspected Chinese food syndrome).
What material is required for blood collection?
Required are 4 mL fresh EDTA or 10 mL heparin blood.