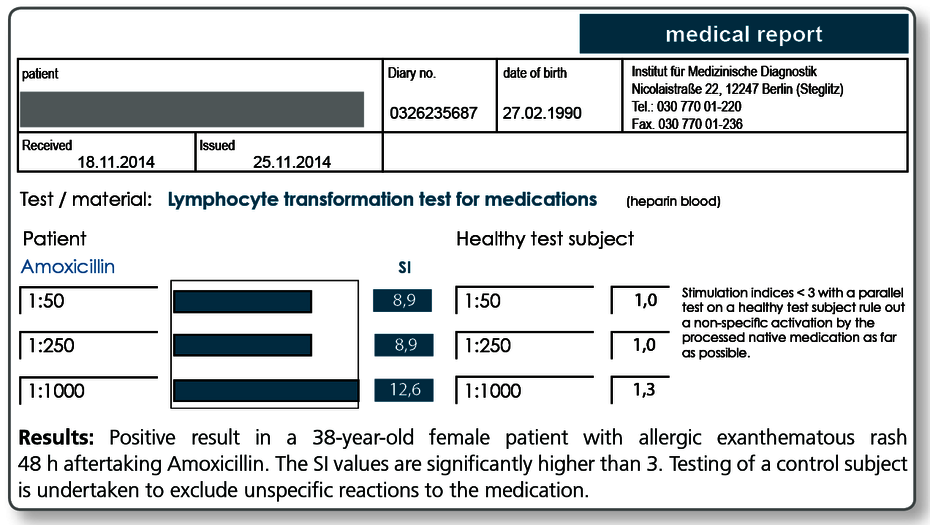
LTT - Medications
The lymphocyte transformation test (LTT) in laboratory testing for drug allergies
“Since skin tests and in vitro IgE testing in patients with exanthematous rashes after medication intake often do not yield any useful results, exanthematous drug eruptions are among the most common indications for requesting an LTT to fill this diagnostic gap.“
In-vitro allergy testing guideline of the German Society of Allergology and Clinical Immunology (DGAKI) in agreement with the German Dermatological Society (DDG) Journal der Deutschen Dermatologischen Gesellschaft
(2006) 4; 72-85
“The specific LTT to detect an allergic reaction to a medication can be recommended unreservedly where indicated.“
Again from the article “Quality assurance for the lymphocyte transformation test (LTT) – Addendum to the LTT paper of the committee for “Methods and Quality Assurances“ at the Robert Koch Institute, published in Bundesgesundheitsblatt 2008 RKI; 51: 1070-1076.
When should the LTT be requested?
A type IV drug allergy can be detected with the greatest accuracy using the LTT between approx. 2 weeks and approx. 3 months after the allergic manifestations have cleared.
However, positive results can still be found years later without any further (known) exposure. The fading sensitivity over time is attributed to the fact that the number of T cells circulating in the blood decreases without continued exposure. The low sensitivity during the phase of acute allergic manifestation (dermatitis, hepatitis during the first 2 weeks afterwards) is explained by the fact that the responsible T cells are staying in the affected organ (here skin or liver) and are there responsible for the local inflammation.
What sampling material is required the LTT Medications?
- Per medication, 5 mL of heparin blood is required.
- In case of 3 or 4 medications: 2 heparin Monovette blood collection tubes (20 mL heparin blood and 5 mL whole blood).
The laboratory stocks a number of medicines and active ingredients. To request current list, please call Tel. +49 30 77001-220.
Alternative, a tablet (or ampoule) of the suspected product can be send in together with the blood samples. This ensures that the administered (batchidentical) product can be tested.
Literature
- Anliker MD et al. (2003) Acute generalized exanthematous pustulosis due to sulfamethoxazol with positive lymphocyte transformation test (LTT). J Investig Allergol Clin Immunol.;13 :66-8.
- Basketter D. et al. (2005)Lymphocyte transformation test in patients with allergic contact dermatitis. Contact Dermatitis;53:1.
- Constable S et al. (2006) Systemic illness with skin eruption, fever and positive lymphocyte transformationtest in a patient on irbesartan. Br J Dermatol.;155:491-3.
- Cooper HL. Et al. (2008) Antibiotic hypersensitivity mimicking recurrent endocarditis-identifying the culprit with the in vitro lymphocyte transformation test. QJM; 101: 67-8.
- Hagemann T. et al. (2005) Positive lymphocyte transformation test in a patient with allergic contact dermatitis of the scalp after short-term use of topical minoxidil solution. Contact Dermatitis; 53: 53-5.
- Jurado-Palomo J. Et al. (2010) Use of the lymphocyte transformation test in the diagnosis of DRESS syndrome induced by ceftriaxone and piperacillin-tazobactam: two case reports. J Investig Allergol Clin Immunol.;20:433-6.
- Kano Y. (2007) Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: dependence on its timing and the type of drug eruption. Allergy; 62: 1439-44.
- Kanny G et al. (2005) T cell-mediated reactions to iodinated contrast media: evaluation by skin and lymphocyte activation tests. J Allergy Clin Immunol.;115: 179-85.
- Kardaun SH . et al. (2006) Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol.; 55: S21-3.
- Kim MH et al. (2012) A case of allopurinol-induced fixed drug eruption confirmed with a lymphocyte transformationtest. Allergy Asthma Immunol Res.;4:309-10.
- Merk HF (2005) . Lymphocyte transformation test as a diagnostic test in allergic contact dermatitis. Contact Dermatitis.; 53: 246-51.
- Merk HF. (2005) Diagnosis of drug hypersensitivity: lymphocyte transformation test and cytokines. Toxicology.; 209: 217-20. Review.
- Nyfeler B. et al. (1997) The lymphocyte transformation test for the diagnosis of drug allergy: sensitivity and specificity. Clin Exp Allergy.; 27: 175-81.
- Pichler WJ. (2005) Direct T-cell stimulations by drugs--bypassing the innate immune system. Toxicology; 209: 95-100.
- Pichler WJ. Et al. (2004) The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy.;59 :809-20. Review.
- Piñero Saavedra MC et al. (2011) Usefulness of lymphocyte activation test in atorvastatin hypersensitivity. J Investig Allergol Clin Immunol. ; 21: 574-5.
- Romano A. et al. (2011) Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 127: 67-73.
- Sachs B. et al. (2000) Drug-induced allergic cytopenia: in vitro confirmation by the lymphocyte transformationtest. Arch Intern Med. ;160: 2218-9.
- Schreiber J. et al. (1999) Lymphocyte transformation test for the evaluation of adverse effects of antituberculousdrugs. Eur J Med Res.; 25:67-71.
- Clin Exp Allergy. 1997 Feb;27(2):175-81.
- Wolf R. et al. (2005) Lymphocyte transformation test in patients with allergic contact dermatitis. Contact Dermatitis.;53 :245-50.