Coeliac disease
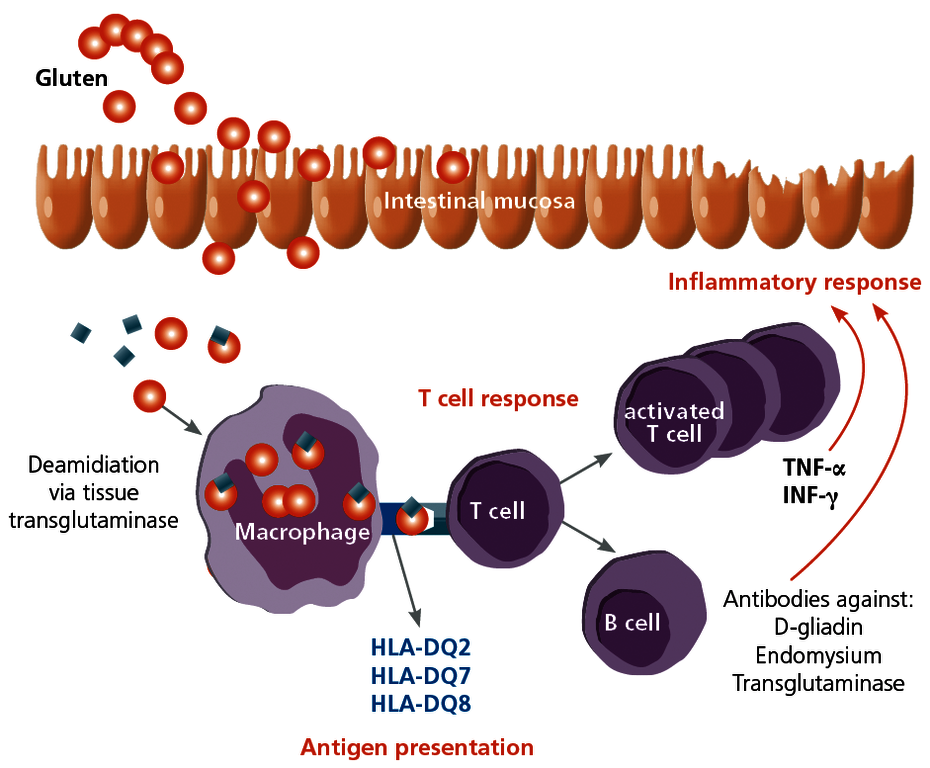
Coeliac disease is an autoimmune disease that is triggered by gluten, which is contained in many types of cereals (wheat, rye, barley, etc.). It occurs in genetically predisposed individuals and results in a lifelong enteropathy. It is thus not an allergy. The autoantigen in coeliac disease is the tissue transglutaminase of the small intestine in the complex with the gluten ingested through food. Since only HLA molecules DQ2, DQ7 or DQ8 can bind to gluten and present it to the immune system, only carriers of these HLA types are affected by the disease. With a prevalence of 1:200–1:500, the disease is not uncommon.
The gluten-induced chronic inflammation of the mucous membrane in the small intestine causes varied clinical symptoms.
Patients suffer from chronic inflammation of the mucous membrane in the small intestine, villous atrophy, and crypt hypertrophy. The resulting clinical manifestations range from asymptomatic or mild through to severe forms. The patients with typical symptoms represent only the tip of the iceberg. Coeliac patients can develop the following symptoms:
| General | weight loss, tiredness, failure to thrive, anaemia |
|---|---|
| Gastrointestinal tract | bloated stomach, diarrhoea, nausea, stomach pain, vomiting |
| Muscular and skeletal systems | arthralgia, myalgia, osteoporosis, seizures |
| Skin and hair | oedema, dermatitis herpetiformis, alopecia |
| Oral cavity | aphthous stomatitis, enamel hypoplasia, burning tongue |
| Endocrine system | Infertility, repeated miscarriage |