Laboratory diagnostics for periodontal diseases
Periodontitis
Perceptions about the pathogenesis of periodontitis have changed considerably in recent years. While previously it was considered a purely microbiological problem (plaque hypothesis), we now know that the individual immune response of a patient is critical for determining the consequences of the trigger stimulus (biofilm) for the patient. In most cases, progressive periodontitis is caused by a periodontal inflammation system that responds excessively to bacteria (referred to as high responders). In the remaining cases, immunodeficiencies, which are associated with reduced resistance of the mucous membrane, can also lead to persistent inflammations because the body’s defence against pathogens is rather too weak.
While the high responders (tendency to develop chronic inflammation) can respond successfully to anti-inflammatory therapy and for whom immunostimulation is rather contraindicated, for patients with a reduced defence against pathogens on the other hand measures that support the immune system have priority (antimicrobial therapy, if applicable, immunostimulation with interdisciplinary cooperation). An anti-inflammatory (= immunosuppressive) therapeutic approach would be contraindicated here.
To clarify the cause and make a prognosis as well as to determine the choice of accompanying therapy, the following laboratory tests are available:
- Detection of high responders
Determination of the genetic predisposition to inflammation (IL-1A, IL-1B, IL-1RN, TNF-A, where applicable also IL-6, IL-10) - Detection of reduced defence against pathogens
Serum IgA, MBL, granulocyte function - Microbiological investigation of the subgingival flora
Detection of periodontitis-associated marker microbes - Assessment of periodontal tissue destruction
aMMP-8
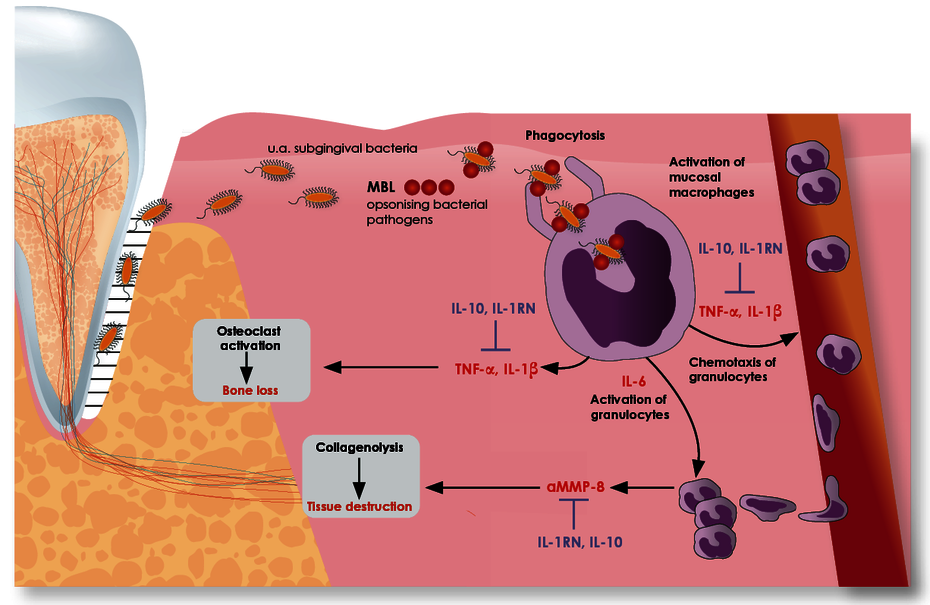
The ratio of pro- and anti-inflammatory cytokines determines the course of the periodontal inflammatory response.
The stimulus that triggers periodontal inflammation is the subgingival (periodontitis) or supragingival (peri-implantitis) biofilm. The macrophages activated by this biofilm secrete pro-inflammatory cytokines, particularly interleukin-1 (IL-1), tumour necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). These cytokines activate osteoclasts which leads to increased alveolar bone resorption. They also increase tissue destruction by stimulating granulocytes which release greater quantities of tissue-destroying matrix metalloproteinase 8 (aMMP-8). To prevent excessive (chronic) inflammation, the inflammatory response is normally later slowed down by the anti-inflammatory cytokines IL-1 receptor antagonist (IL-1RN) and interleukin-10 (IL-10).
The course of the periodontal inflammatory response is thus determined by the ratio of pro- and anti-inflammatory cytokines. The amount of each of these cytokines that is released as part of the immune response is individually determined by variants in the genes for these cytokines. Pro-inflammation predominates in about 15% of the population on the basis of genetics.
Four polymorphisms determine the genetic degree of inflammation of a patient.
Because the polymorphisms for the pro-inflammatory cytokines IL-1α, IL-1ß and TNF-α lead to an increased synthesis of these mediators, their presence results in an amplified inflammatory response. If the polymorphism in the gene for the IL-1 receptor antagonist (IL-1RN) is also present, this is released in smaller quantities, that is, inhibition of inflammation is also unfortunately absent.
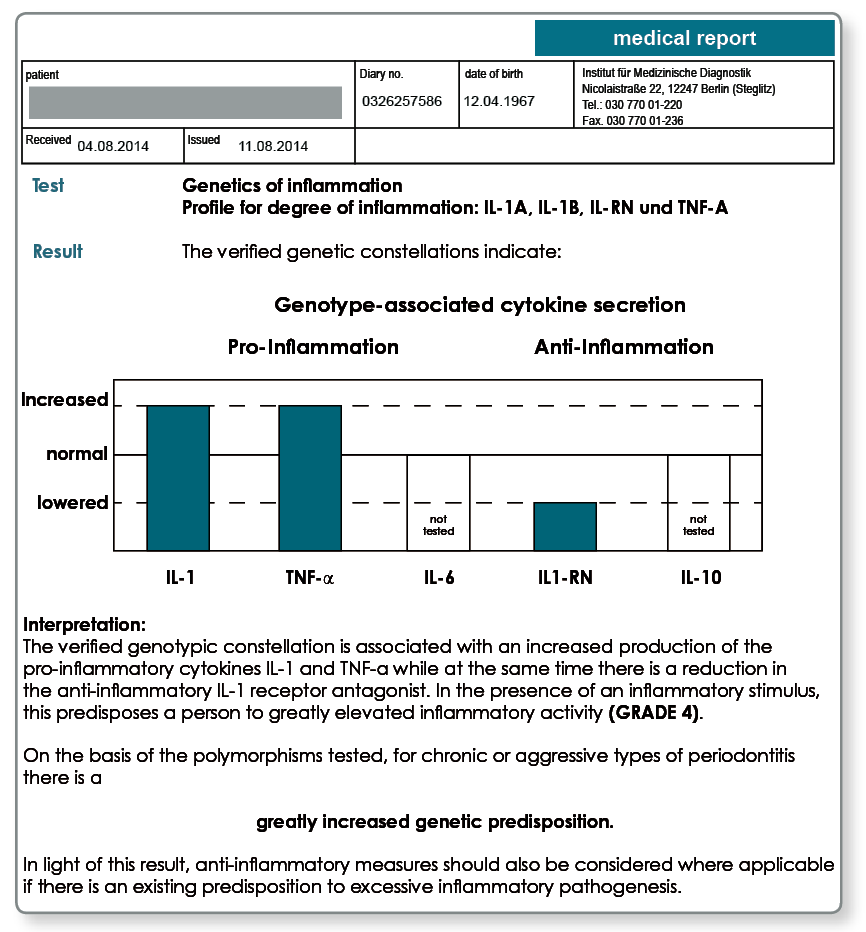
The exceptional importance of the above cytokines for the individual inflammation susceptibility led to grading of the inflammation susceptibility using the genetic constellations described. The genetic degree of inflammation increases depending on the number of verified polymorphisms present from grade 0 (no polymorphism present) to grade 4 (all four polymorphisms tested are present).
Patients with a degree of inflammation of 0 and 1 are referred to as low responders because they have a normal inflammatory capacity. Patients in grades 2 to 4, the high responders, have a greatly elevated susceptibility to inflammation that is genetically determined.
Notes on the results: Periodontitis genetics results for a patient with generalised periodontitis. A degree of inflammation of grade 4 was verified. With this predisposition to chronic types of inflammation, a concomitant anti-inflammatory therapy is recommended.
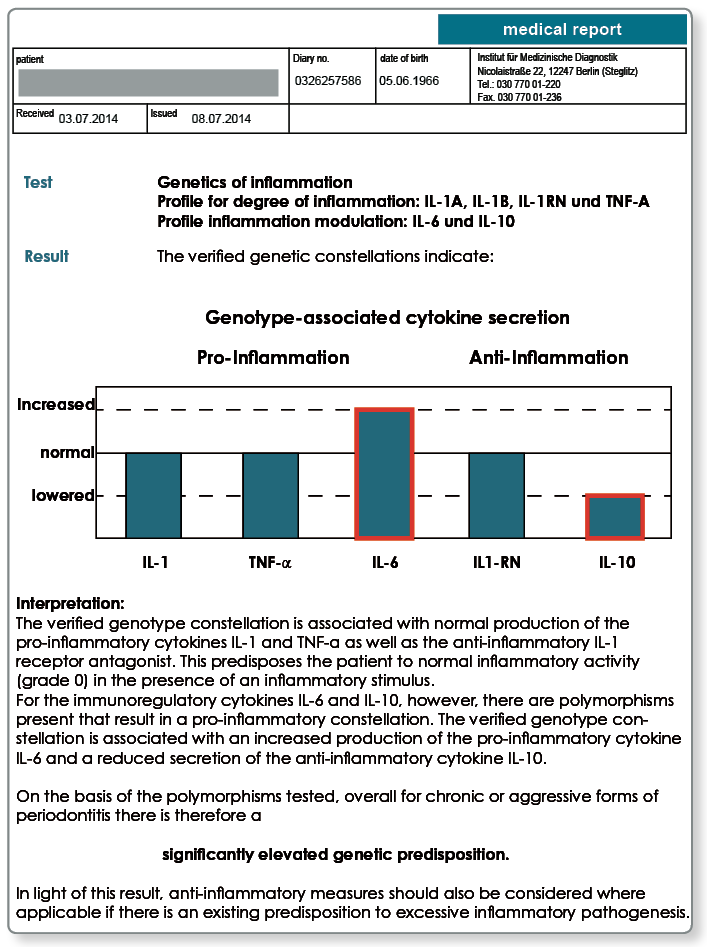
Polymorphisms in the IL-6 and IL-10 genes supplement the diagnostics for problematic cases.
Even if the extent of the initial inflammatory response is primarily determined by TNF-α, IL-1 and IL-1RN, it may be that genetic variants in the cytokines IL-6 and IL-10 lead to a high responder status.
The pro-inflammatory cytokine IL-6 is secreted by macrophages and epithelial cells amongst others and, like IL-1 and TNF-α, increases tissue destruction and bone resorption. On the other hand, IL-10 is an anti-inflammatory cytokine which, like IL-1RN, counteracts bone loss and tissue destruction caused by inflammation.
While the polymorphism in the IL-6 gene causes an increase in the release of IL-6, the polymorphism in the IL-10 gene is associated with reduced levels of IL-10. If both these genetic variants are present, similar to a patient with a degree of inflammation of grade 3 or 4, the result can be elevated pro-inflammation (increase in the release of IL-6) with lowered anti-inflammation (decrease in the release of IL-10).
What is the diagnostic procedure?
For patients with chronic or aggressive periodontitis, the genetic degree of inflammation should first be determined if an excessive inflammatory pathogenesis is suspected. If the test indicates a degree of inflammation of 2, 3 or 4, an elevated inflammation susceptibility is considered as verified. If, however, the test indicates a degree of inflammation of 0 or 1, it is recommended to test the genetic IL-6/IL-10 variants in a second step. If the IL-6 and IL-10 polymorphisms are present, then there is an increased tendency towards inflammation despite a normal genetic degree of inflammation.
Notes on the results: The test of the genetic inflammation susceptibility initially indicated a normal result (grade 0). The subsequent test of the IL-6 and IL-10 polymorphisms revealed a high responder status, however.
What should be noted for periodontitis patients with an elevated inflammation susceptibility?
If the genetic inflammation susceptibility is verified as being elevated, this leads to the following risks and therapeutic consequences:
- For these patients, it is not uncommon that following antibiotic and/or manual therapy the marker microbes results temporarily decline but the tissue destruction marker aMMP-8 remains elevated and the clinical signs of inflammation persist. This is often followed by recolonisation by the marker microbes and thus the development of refractory periodontitis.
- As well as local manual therapy, a concomitant anti-inflammatory therapy should also be selected.
- What is important is that these patients do not receive immunostimulatory therapy.
- Sensitisations to dental restoration materials and other odontogenic interference factors should be specifically searched for and eliminated.
- An awareness of a genetic predisposition to a progressive course for periodontitis should help and be a reason to motivate the patient to stop smoking and maintain oral hygiene. Short intervals between recalls and prophylaxis are also recommended.
Sample materials required
2 mL EDTA blood or 2 buccal swabs.
Transport to the laboratory is not time critical and can be done by post.
For the genetic tests we require a declaration of consent of the patient. All the polymorphisms indicated can be tested using a single sample.
Reduced mucosal resistance as the cause of chronic periodontal disease
The expansion and translocation of periodontal pathogenic bacteria into the periodontium is controlled primarily by granulocytes. The prerequisites for this are detection and binding (opsonisation) of the pathogens. Attachment of the body’ proteins (antibodies, complement factors, MBL [mannose-binding lectin]) to the microorganisms improves identification of the microorganisms by the phagocytic cells.
Particularly in cases of chronic periodontitis, which is not due to an elevated inflammation susceptibility, defects in these immune defence mechanisms (opsonisation, phagocytosis) may be the cause. For differential diagnosis of a reduced mucosal resistance, the following tests are recommended:
IgA
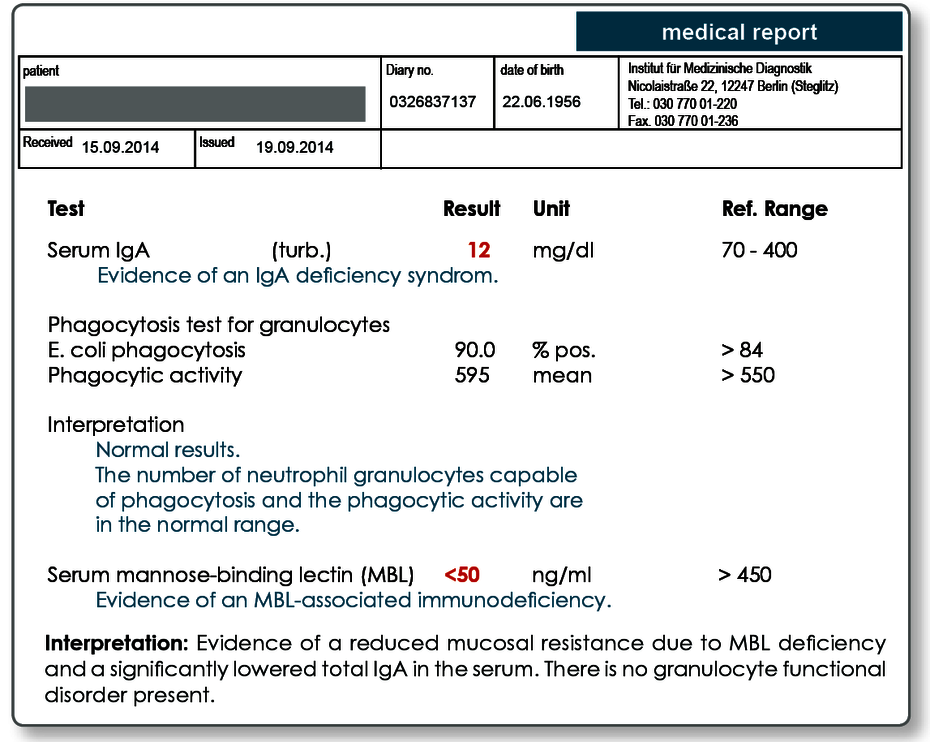
IgA plays a central role in the defence against infections of the mucous membranes. Selective IgA deficiency is common with a prevalence of 1:250 to 1:500.
MBL
Another important component of the innate or non-specific immunity is MBL (mannose-binding lectin). It has a high affinity for the repetitive carbohydrate compounds containing mannose found on the surface of bacteria and fungi.
Using these compounds, it binds the periodontal pathogenic bacteria and facilitates their phagocytosis. This leads to rapid and efficient elimination of the pathogens in the periodontium, which in turn limits any increase in the inflammatory response. The prevalence of an MBL deficiency is about 2–5% in our population.
Granulocyte function
In rare cases, functional defects in the granulocytes may also be responsible for chronic periodontitis. Generally, these are secondary phagocytosis defects which may be the result of metabolic disorders (e.g. diabetes) or chronic inflammatory diseases.
What should be noted for periodontitis patients with a reduced mucosal resistance?
For patients with reduced defence against pathogens, measures that support the immune system should have priority (antimicrobial therapy). In cases of chronic periodontitis in which there is no increased inflammation susceptibility, an additional immunostimulation (where applicable, with interdisciplinary cooperation) would be another possible therapeutic approach. On the other hand, an anti-inflammatory (= immunosuppressive) therapeutic approach would be contraindicated for these patients.
Sample materials required
MBL and IgA are measured in the serum. One serum tube (10 mL) is sufficient for both tests.
For the granulocyte function test, we require 10 mL heparin blood. For this test, the laboratory must receive the sample within 24 hours of collection. The blood should be stored and transported at room temperature.
Detection of microbes associated with periodontitis
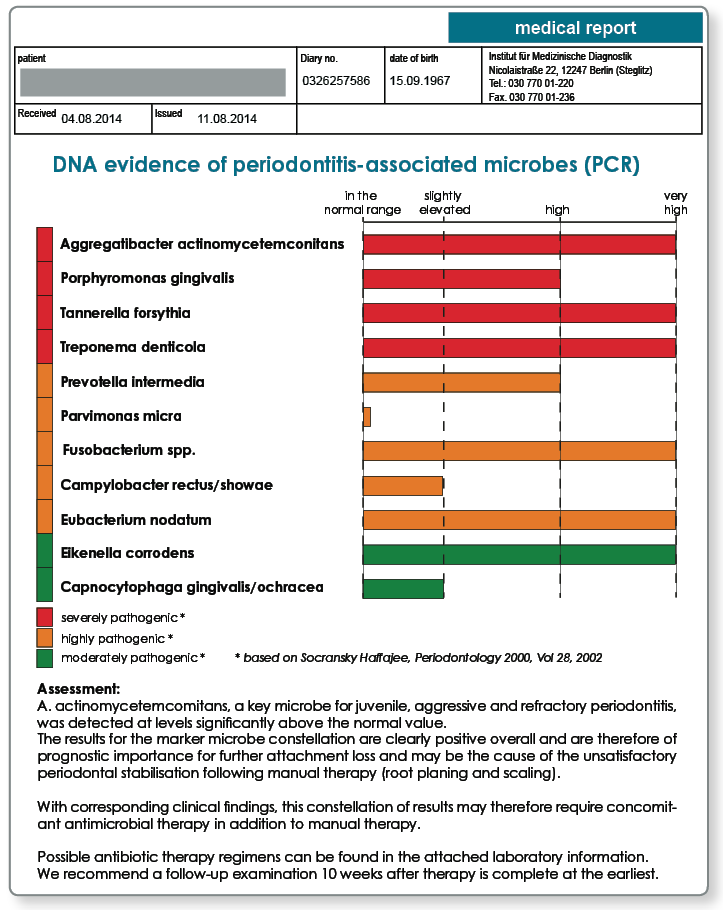
As well as the individual genetic inflammation disposition of the patient, which has a critical influence on the severity of the medical condition, the periodontitis-associated microbes in particular demonstrably shape the inflammatory process. The growth of these anaerobic or facultative anaerobic pathogens is favoured by the lack of oxygen in the periodontal pocket. The periodontal microbes of the local flora are displaced by these anaerobes. If the microbes indicated are present in the sulcus, concomitant antibiotic therapy may be necessary. Active substance and pharmaceutical form are determined by the composition and distribution of the subgingival flora (targeted antibiotic treatment) meaning that a microbial clarification before starting therapy is helpful.
The microbiological conditions can be reliably determined using a molecular biology test.
While clinical diagnostics in the past were restricted to the most important key microbes Aggregatibacter actinomycetemcomitans, Prevotella intermedia, Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, we are able to cost-effectively carry out semi-quantitative assessments of the equally important microbes Campylobacter rectus/showae, Eikenella corrodens, Capnocytophaga gingivalis/ochracea, Parvimonas micra, Eubacterium nodatum and Fusobacterium spp. using modern chip technology and to integrate these into the therapeutic approach.
The decisive advantage of molecular biology verification compared to bacterial culture methods is its sensitivity because anaerobic bacteria often die during transport to the laboratory. The PCR-based chip procedure is equally able to detect the DNA of living and dead pathogens, however.
When is determination of periodontitis-associated microorganisms indicated?
The marker microbes mentioned represent an immunological focus and must be considered as potential inflammatory stimuli for therapy resistant, refractory and acute, rapidly progressing periodontitis along with peri-implant infections.
Determination of the marker microbes provides important information in periodontitis diagnostics and treatment as well as implantology and prosthetics:
- Evidence of the key microbe for ‘aggressive periodontitis’: Aggregatibacter
- Indication for antibiotic therapy and choice of preparations
- Monitoring success of the therapy
- Identification of risk points
- Determination of the risk of recurrence
- Estimation of the risk of tissue destruction prior to extensive prosthetic restorations, e.g. prior to placement of implants
- Diagnosis of peri-implant inflammations
What should be done if the inflammation persists clinically after elimination of the marker microbes?
The anaerobic marker microbes mentioned represent the causal factor (inflammation stimulus) for periodontitis. Ultimately, however, the body’s own inflammatory response is responsible for the destruction of the tooth support structure and this varies enormously from patient to patient depending on the individual’s genetically determined inflammation susceptibility (see page 50). For patients with refractory periodontitis but also for patients who show clinical signs of persistent inflammation after elimination of verified marker microbes, an excessive inflammatory response is the cause in many cases. For these high responders (usually with a genetic degree of inflammation of grades 3 and 4), along with the elimination of the marker microbes (stimulus elimination), an anti-inflammatory but never an immunostimulatory therapy is indicated. In contrast, elimination of the trigger stimulus (including the marker microbes) is the top therapeutic priority for normal responders. For these pathogen-induced processes, additional immunostimulation would then make sense, unlike the situation for high responders.
Sample materials required
The sample is collected from the dental pocket using paper strips which are included in the relevant collection set. The collection must be done prior to any mechanical treatment or antibiotic therapy. Collection sets are provided by the laboratory.
Literature
aMMP-8 – a biomarker for periodontal tissue destruction with periodontitis
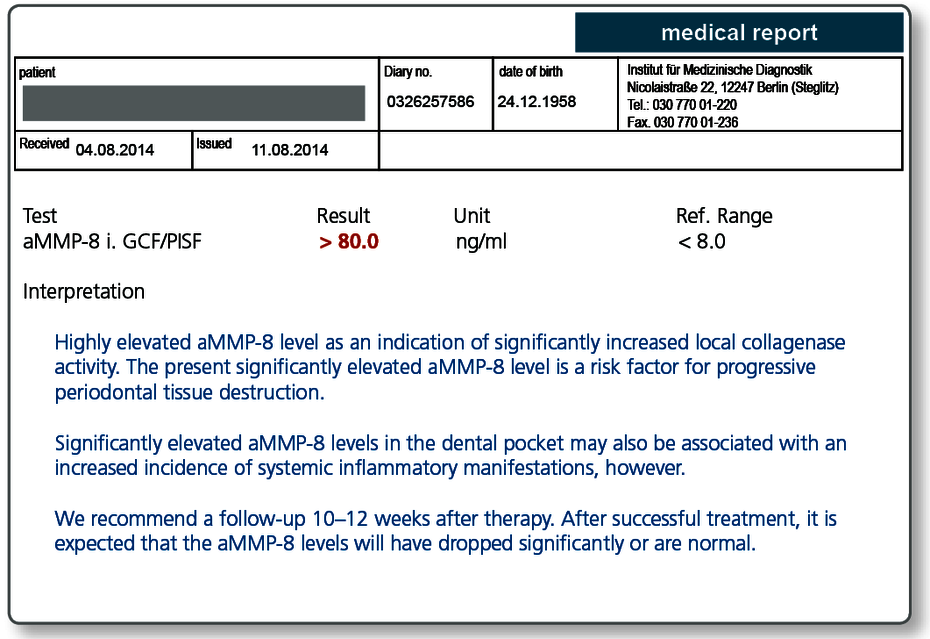
Determining the periodontal screening index (PSI) and radiology are established diagnostic procedures for periodontology. However, these two procedures only document any soft tissue and hard tissue destruction afterwards and therefore very late. Quantitative detection of the active collagenase aMMP-8 (matrix metalloproteinase-8) in the sulcus fluid is an innovative test for early diagnosis of periodontal or peri-implant tissue destruction.
aMMP-8 is an inflammatory marker that can be directly measured at the site of the periodontal inflammation.
Periodontal pathogenic bacteria in the dental biofilm that are taken up by tissue macrophages trigger the inflammatory cascade in the pathogenesis of periodontitis. As described on page 49, the resultant activated macrophages initiate the inflammatory cascade as part of which infiltrated granulocytes release the enzyme MMP-8. As a collagenase, MMP-8 (previously referred to as collagenase-2) in its active form destroys the three-dimensional collagen network of the periodontium to facilitate infiltration of additional cells of the immune system.
The enzyme aMMP-8 is therefore primarily responsible for tissue destruction. Measuring the aMMP-8 level in the sulcus fluid therefore enables early detection of attachment loss and thus timely therapeutic intervention.
aMMP-8 determination is considered a progression marker.
aMMP-8 determination provides information about the inflammatory status of the periodontium often before clinical signs become apparent. Because aMMP-8 is not only an inflammation marker but also a direct marker of tissue destruction, it can deliver precise information about the medium-term progression.
The aMMP-8 levels fall within 10–12 weeks back to the normal range after successful periodontal treatment. The aMMP-8 levels therefore also enable detection of refractory disease.
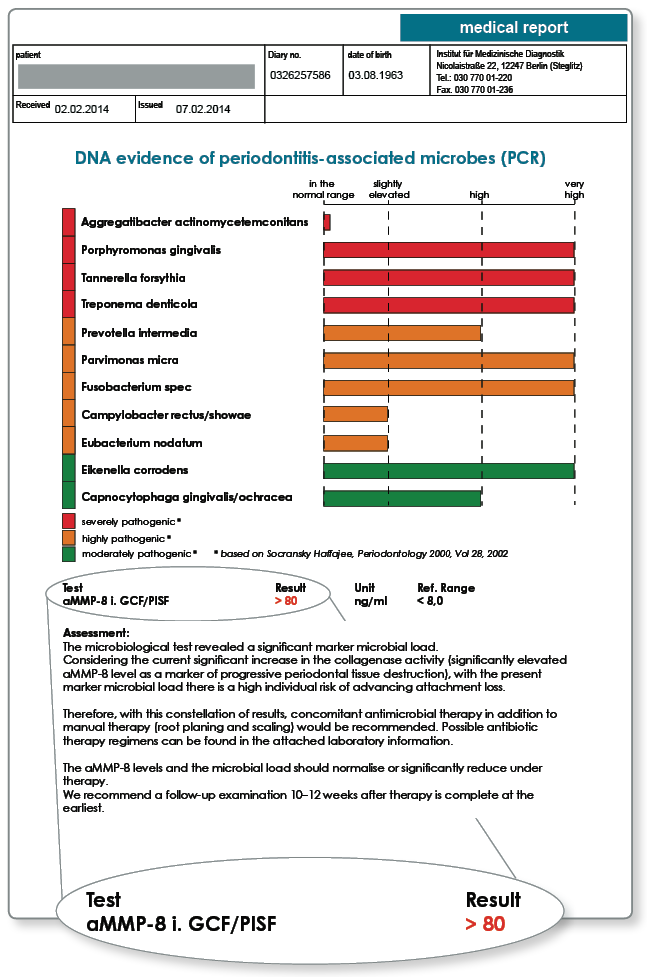
Notes on the results: Marker microbe diagnostics and aMMP-8 determination in a patient with generalised periodontitis. Subsequently, antibiotic therapy (according to Winkelhoff) was applied along with curettage and root planing.
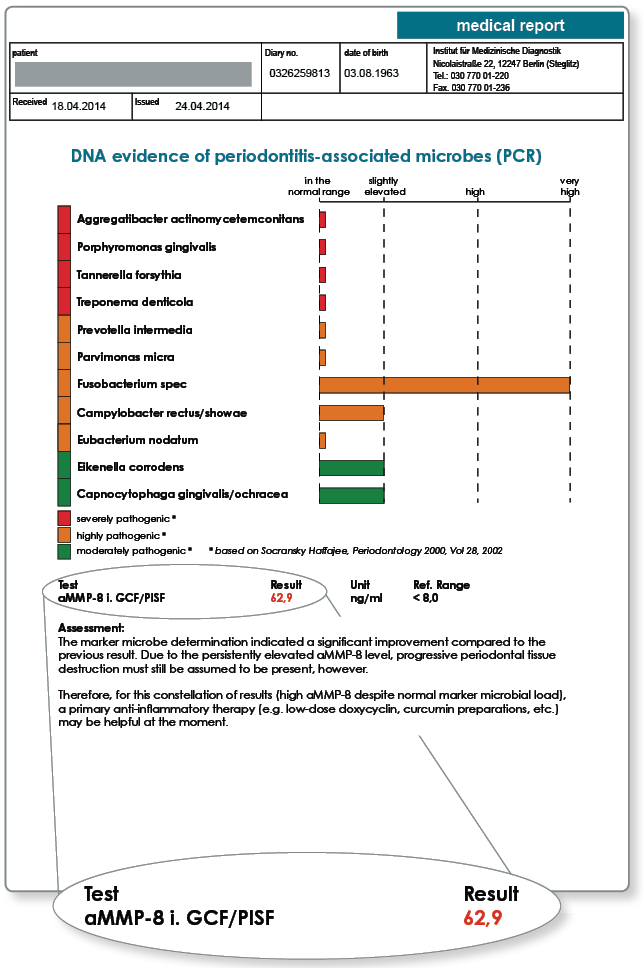
Notes on the results: The follow-up examination reveals that the marker microbial load has declined significantly as a result of the periodontal treatment. Further primary antimicrobial therapy is therefore no longer indicated. The aMMP-8 level allowed an objective evaluation of the remaining enzymatic activity and the associated inflammatory activity. The aim of the therapy should be an aMMP-8 level in the normal range or at least a clearly falling aMMP-8 level because there is a risk of recolonisation by the anaerobic bacteria in still inflamed tissue. Therefore, for this constellation of results a primary anti-inflammatory therapy may be very helpful. The persistently high aMMP-8 also requires a short recall interval and interdisciplinary care where appropriate.
aMMP-8 is also an early marker for peri-implantitis /mucositis
Determination of aMMP-8 levels in the peri-implant sulcus fluid (PISF) can be used for routine monitoring of the implant or early detection of mucositis or to promptly initiate therapeutic measures for suspected peri-implantitis / mucositis.
The aMMP-8 level in the PISF correlates in these cases with bone loss. With peri-implantitis the aMMP-8 levels are increased up to 1000-fold compared to a healthy implant. Another indication is for monitoring the local inflammatory status prior to placing an implant.
The higher the aMMP-8 in the dental pocket is, the more likely systemic manifestations are.
The epidemiological associations between periodontitis and cardiovascular diseases, the risk of stroke, diabetes mellitus, increased danger of premature births and low birth weight for pregnant women have all been established.
Determining the aMMP-8 is a valuable tool for diagnostics, particularly for these coincidental associations. Numerous studies show that patients with elevated aMMP-8 in the dental pocket have an increased risk of developing these systemic complications.
Regardless of the dental indication (early detection of progressive periodontitis), regularly determining the aMMP-8 level is therefore recommended for all patients with the systemic diseases mentioned above.
If the values are elevated, the therapeutic measures should be applied more intensively. An elevated aMMP-8 level should also mean that the patient receives joint interdisciplinary care from other medical specialists.
Sample materials required
To detect the aMMP-8, a sampling strip included in the collection set is inserted into the sulcus and sent to the laboratory in a shipment tube by post.
The collection and shipping material is provided by the laboratory free of charge.
Literature