Loading with metals
Salivary metal exposure due to dental restorations
Depending on their composition, condition, location and size, metallic dental restoration materials can release various amounts of metals into the saliva and the surrounding tissues through wear and corrosion. In individual cases, this can cause a local or even a generalised inflammation of the gums. Because metals in the saliva end up in the gastrointestinal tract after swallowing, the mucous membranes of the gastrointestinal tract are also exposed and may also become irritated.
Beyond their local effect, permanently elevated intestinal exposure to metals increases the risk of developing systemic physical stress. Thus chronic metal exposure is considered a trigger factor for the development of many chronic inflammatory diseases. The link with chronic fatigue syndrome, hypertension and neurological disorders has been well established. Along with the magnitude of the metal exposure, the individual susceptibility of the patient is also important for the type and degree of the symptoms.
Toxic metal effects may develop even with a negative LTT
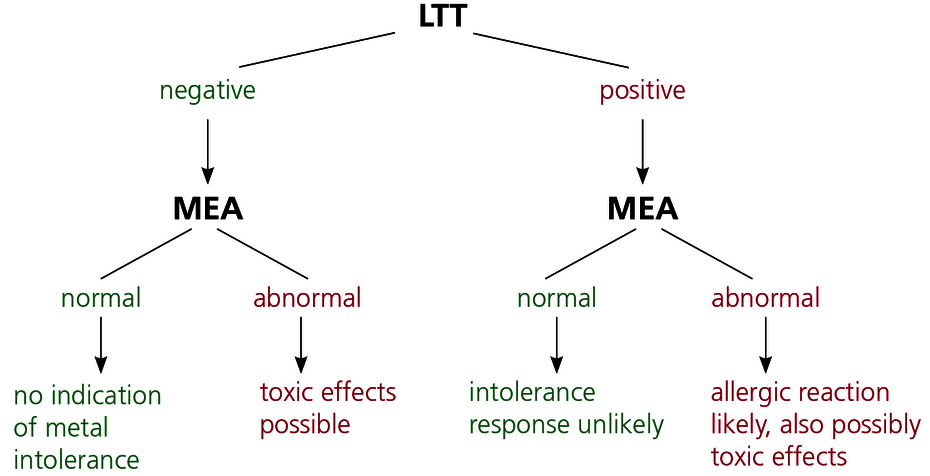
Metals exert their effects on the body in two ways. For individual existing sensitisations (detected using the LTT), even very small loads can induce a type IV immune response. The permanent immune activation resulting from persistent exposure can include all aspects of a chronic inflammation such as increased local inflammatory processes, autoimmune reactions and fatigue. Diagnosis of type IV allergy is described in detail.
At the same time, however, metals such as mercury, cadmium and palladium have toxic effects even in low doses and inhibit cellular metabolic processes. Even very low (subtoxic) concentrations are also possibly of clinical relevance because multiple sources of exposure (from food and drinking water as well) can potentiate the toxic effect of the individual metal. Toxic effects also occur without an existing allergic sensitisation. This means that a negative LTT result means toxic exposure is possible but a positive LTT also means that any additional metal exposure may be clinically significant.
What type of saliva sample is submitted is determined by the indication!
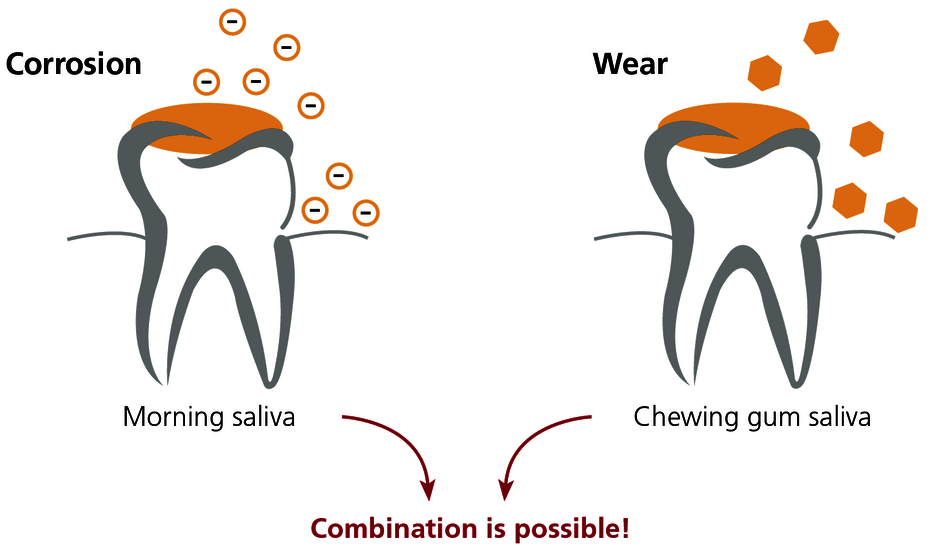
Morning saliva is used for sensitive detection of corrosion. Due to the reduced salivary flow overnight, the ions released accumulate and are present in higher concentrations than during the day. Exercise caution with bruxism: nocturnal grinding releases wear particles which leads to high levels of metal in the morning saliva independent of corrosion.
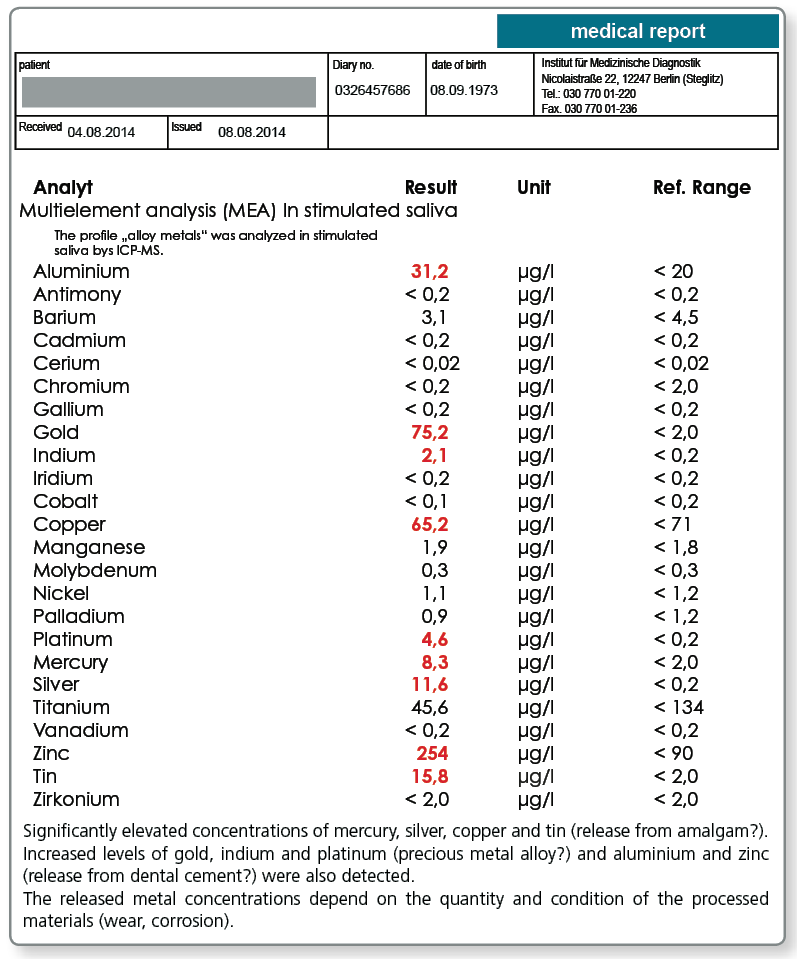
Chewing gum saliva is used for sensitive detection of mechanical wear of metal particles from the dental restoration. Chewing gum for 10 minutes stimulates wear and the saliva sample is then collected. Wear is seen predominantly from ‘soft’ gold alloys (e.g. BioGold) and amalgam.
The combined saliva is usually selected in practice because differentiating between corrosion and wear is often not a priority. Morning saliva and chewing gum saliva are collected, preferably in an equal ratio, in the same collection tube. The analysis of the combined saliva indicates the total metal exposure for the patient through his or her saliva.
Salivary metals do not originate just from dental alloys!
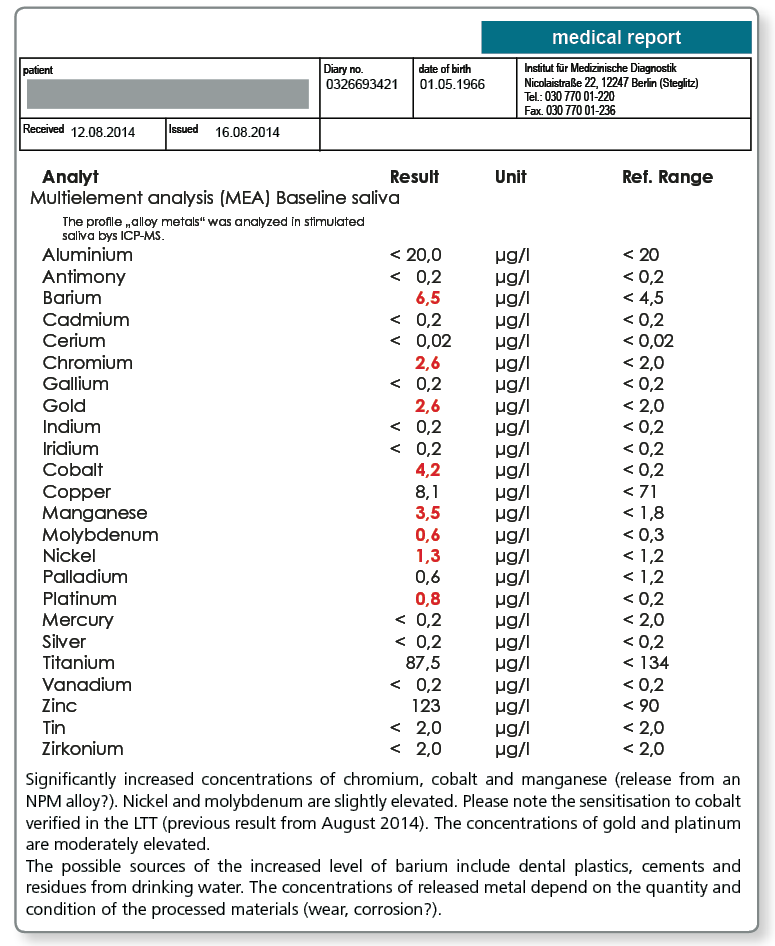
Studies show that metal released into the saliva can also occur from soldered joints (silver, copper), dental cements (aluminium, antimony, zinc) and even (usually under-fired) ceramics (aluminium, barium, zinc). As for the release from alloys, the pH of the oral cavity is again an important factor influencing the release. It has been observed that residues from teeth cleaning products (titanium, tin) and food and drink (e.g. barium in drinking water) can also be measured, particularly in the morning saliva. Some alloy metals are also present in the saliva as physiological electrolytes (copper, manganese, zinc). Shifts in the electrolyte balance can therefore be superimposed over the exposure from dental restorations.
ICP-MS is the most sensitive analytical procedure.
Multi-element analysis (MEA) using ICP-MS (inductively coupled plasma mass spectrometry) provides the option of simultaneously detecting a range of metals in a saliva sample. The sensitivity is considerably better compared to the conventional procedure of atomic absorption and extends to the ng/L concentration range. For the measurement, the sample is nebulised to form ultra fine droplets which are then decomposed into their elemental components in an argon gas plasma jet at 8000°C. The resulting metal ions are then analysed in a connected mass spectrometer.
Sample materials required
3–5 ml saliva. On request, we will happily send you saliva collection tubes.
| morning saliva | Collection immediately after waking, before breakfast and brushing teeth |
| chewing gum saliva | Collection after chewing gum for 10 minutes |
| combined saliva | Collection of morning saliva and chewing gum saliva in the same tube |
Literature
Systemic symptoms due to systemic metal exposure?
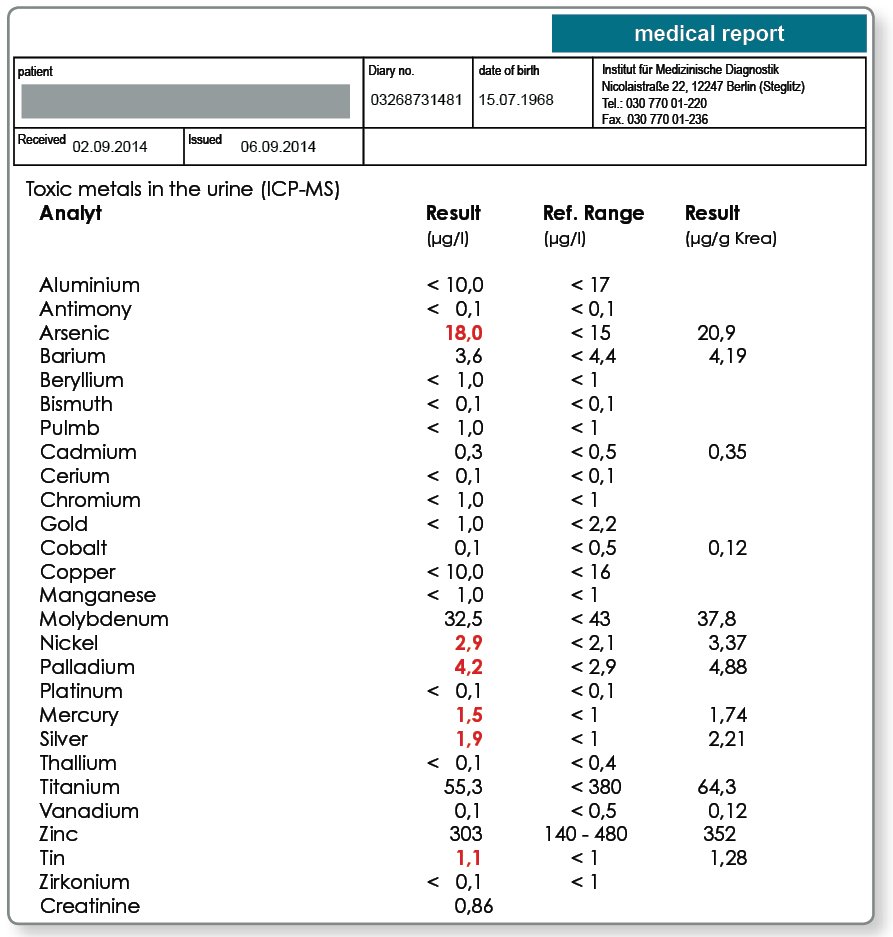
Systemic exposure of the body must be strictly differentiated from exposure in the oral cavity because the intestine only absorbs some of the metals from the swallowed saliva. The question of systemic metal exposure from dental restorations is particularly relevant when a causal relationship to systemic symptoms is suspected. Because corroding or heavily wearing dental restorations are a permanent source of exposure, systemic uptake of the released metals can be very easily measured using multi-element analysis in the morning urine or EDTA blood. Note that the systemic exposure originates from many other sources in addition to the dental restoration such as food, drinking water, air and possible endogenous sources (e.g. endoprostheses).
Results:
Indication of systemic exposure to arsenic, nickel, palladium, mercury, silver and tin. Metal exposure can originate from a range of sources. The following information should provide information to identify the individually relevant sources of exposure and represent possible biological effects of the metal exposures detected. In principle, many metals trigger dose-dependent inflammatory processes in the endothelium and immune cells and by inducing oxidative stress they can damage cellular membranes, proteins and DNA. We do not claim that the information is complete and it does not replace a clinical analysis of the laboratory results by the treating doctor.
Arsenic:
Important sources of exposure include: fish, seafood, seaweed, waste incineration, tobacco smoke, fruit cultivation, imported wines. Systemic biochemical effects of exposure may include: arsenic inhibits the production and activity of ATP; blocks DNA repair (Gentry et al., Environ Mol Mutagen 2010; 51:1–14).
Nickel:
Important sources of exposure include: nuts, bananas, coffee, cocoa, chocolate, drinking water (particularly after standing in tap fittings), dental materials, dental solders, endoprostheses, fashion jewellery, piercings, tattoo dyes, cosmetics, textile dyes, cutlery, saucepans, coffee machines, industrial emissions, tobacco smoke, toner powder. Systemic biochemical effects of exposure may include: damage to DNA due to displacement of magnesium from the heterochromatin, modification of the epigenetic character, activation of leukotriene B4 synthesis in granulocytes, induction of allergic sensitisation (Klein et al., Pathobiology 1994; 62:90–8).
Palladium:
Important sources of exposure include: dental alloys, jewellery, dyes, piercings. Systemic biochemical effects of exposure may include: oxidative stress due to glutathione consumption, induction of allergic sensitisation (Mukhtiar et al., Pak J Pharm Sci 2013; 26:131–5).
Mercury:
Important sources of exposure include: amalgam, fish, seafood, energy efficient light bulbs, neon tubes, contact lens cleaner.
Systemic biochemical effects of exposure may include: reduced detoxification capacity due to inhibition of glutathione peroxidase, displaces iron and copper, mitochondrial dysfunction, oxidative stress, can cross the blood-brain barrier after conversion by intestinal bacteria into methylmercury, induction of allergic sensitisation (Farina et al., Neurochem Int. 2013; 62:1–20).
Silver:
Important sources of exposure include: drinking water filters, antiseptics, photo developer, textiles, cosmetics, amalgam and other dental alloys, jewellery.
Systemic biochemical effects of exposure may include: reacts with thiol groups and other functional groups of proteins/enzymes, destroys cell membranes, disrupts mitochondrial metabolism, induction of allergic sensitisation (Garcia-Reyero et al., Environ Sci Technol. 2014; 48:4546–55).
Tin:
Important sources of exposure include: metal cans, fashion jewellery, amalgam and other dental alloys, dental cements, dental cleansers (tin fluoride), paints, toner dust.
Systemic biochemical effects of exposure may include: induction of allergic sensitisation. Highly toxic organic tin compounds cannot be differentiated from less toxic inorganic tin with the method used (Pagliarani et al., Toxicol In Vitro. 2013; 27: 978–90).
The total cumulative exposure can be measured after detoxification.
Particularly with chronic exposure, appropriately trained environmental medicine specialists often prefer multi-element analysis in the urine after administration of chelators (e.g. DMPS, DMSA, EDTA) to baseline urinalysis. Chelators bind heavy metals stored in the tissue and transport them to the renal excretion. A subsequent multi-element analysis of the urine provides information about the total cumulative exposure of the body. The detoxification is therefore simultaneously diagnostic and – thanks to the reduction in the heavy metal exposure of the patient – also curative.
Sample materials required
2 ml EDTA blood or 10 ml urine. On request, we will happily send you collection material.
Biochemical effects of metal exposure include oxidative stress and inflammation.
Even if there is no metal sensitisation present, metal exposure can damage the body. Environmental medicine specialists can study and quantify some of these non-allergic effects using suitable laboratory parameters.
- Metals can trigger chronic immune activation. Unlike a type VI allergy, such inflammatory responses are based on activation of the innate immune system. Useful serum parameters in this regard are the cytokines TNF-α, interleukin-6 and the highly sensitive CRP as markers of inflammation.
- A property common to many metals is their ability to trigger oxidative stress. The free radicals produced attack cellular structures and macromolecules such as DNA which underlies many cytotoxic and mutagenic metal effects. A reliable laboratory parameter for quantifying oxidative stress is malondialdehyde modified LDL (MDA-LDL) in the serum.
- Of great importance because of their toxicity is the interaction between metals and the intestinal mucosa. Metals (from food or dental restorations) can damage the mucosa and increase the permeability of the intestine. An increase in the permeability of the intestinal wall in turn increases the systemic uptake of metals from the intestine. Serum zonulin is a good laboratory parameter for leaky gut syndrome.
Literature