Sensitisation to mercaptans and thioether
Devitalised teeth and the resultant organic intermediary products such as mercaptans and thioether can also act as a focus for immunological inflammatory responses independent of their toxic effects.
Even with almost perfect methods for root canal preparation, it is still not possible to completely remove organic tissue and microorganisms from the root canal. Residual anaerobic bacteria such as Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum or Treponema denticola inevitably produce hydrogen sulfide compounds such as methyl mercaptans and thioether compounds such as dimethyl sulfide and diethyl sulfide. The toxicity of these products has been known for more than 30 years. However, toxic effects alone are generally not able to explain the local and systemic inflammation responses described and the individual variation in the symptoms experienced by patients.
Studies have shown that protein decay products can also cause pathological immune responses in addition to their toxic effects. There is no direct correlation to the dose (that is, to the quantity of toxin) but instead to an individually determined sensitivity which may, however, be amplified by other inflammatory processes.
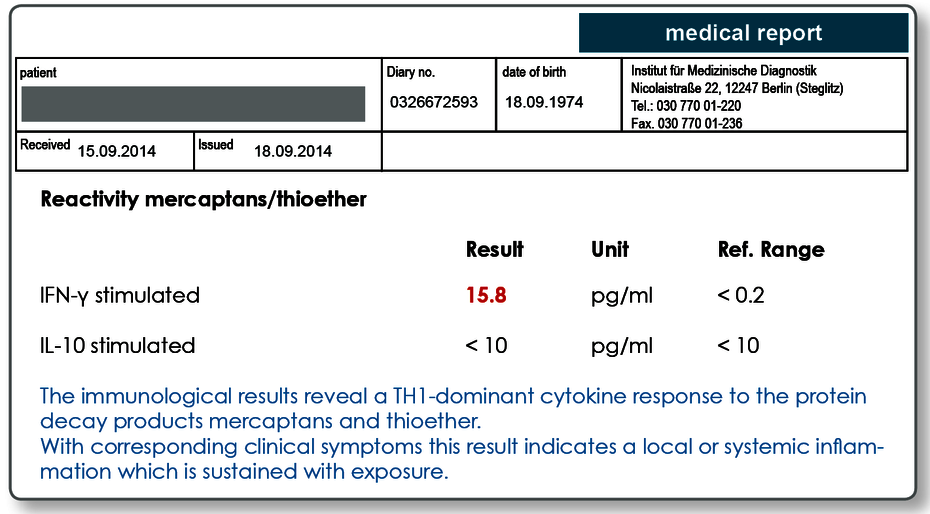
Effector cell typing for mercaptans and thioether verifies an immune response to the substances.
Using the cytokine pattern determined (TH1-IFN-γ / Treg-IL-10), effector cell typing for mercaptans and thioether reveals whether there is currently an immune response to these substances. A positive result indicates both a sensitisation but also makes acute or persistent exposure to mercaptan and thioether compounds probable.
A recently published study (Jacobi-Gresser 2014) not only verified the specificity of the test but it was also demonstrated that the laboratory values were significantly affected by measures such as revision of the root canal filling or tooth extraction. After successful treatment the values measured dropped back to the normal range in more than 90% of patients.
A study into the clinical significance of laboratory tests was conducted in 2013/2014 led by Dr. Jacobi Gresser (Mainz) and published in the Journal of Biological Regulators & Homeostatic Agents in April 2015.
“Methyl mercaptan and hydrogen sulfide products stimulate proinflammatory cytokines in patients with necrotic pulp tissue and endodontically treated teeth”
The main points of the study were:
- The mercaptan/thioether stimulation test is specific to endodontic lesions
Positive results in the mercaptan/thioether stimulation test occurred in 53.4% of patients with clinical/x-ray anomalies in the non-vital tooth (39 of 73 positive, in detail 20 x positive for IFNγ +/- IL-10; 19 x isolated IL-10 positive). In of the control group (patients with non-vital tooth without clinical/x-ray indications), only one of 31 patients showed an isolated positive IL 10-response (3.2%). The group difference was highly significant both for IFNγ and IL-10 (p<0.001). - Remarkable results of the mercaptane/thioether stimulation test decreased after extraction or revision.
Extraction group: Initial value 59.6% positive vs. 14.8% after extraction; Revision group: 52.4% vs. 23.8%). Follow-up occurred after 3 months at the earliest. - The G-308A polymorphism in the TNF-α gene represents a predisposition for the failure of a root canal filling.
The TNF-α polymorphism was detected in 43.8% of patients, but in only 16.1% of the controls (p<0.01). - A normal TNF-a level in the blood does not exclude an endodontic inflammation
Even when the TNF-α blood level was elevated in the patient group in the average value due to the partially existing accompanying systemic inflammation (p<0.05), blood level cytokine analyses of TNF-α or CRP are not suited for use as a screening test due to insufficient sensitivity and by no means exclude a local process.
The study clarified that the test should not be intended primarily to establish a local inflammation. In this case, even qualified imaging diagnostics are more sensitive. Rather, a positive mercaptan/thioether stimulation test reveals that systemic inflammation emanates from the local inflammation, that systemic inflammation manifestations can thus be triggered by the local focus of infection (confirmation of the systemic focus of infection). In addition, follow-up analyses show that the test serves as a valid control instrument of therapeutic measures and progress monitoring.
Sample materials required
10 mL heparin blood. The heparin Monovettes in the LTT collection set can be used. We are also happy to send you individual collection tubes.
The laboratory must receive the samples within 24 hours of collection. The blood should be stored and transported at room temperature.
Literature